The incidence of gestational diabetes mellitus (GDM) has been increasing in multiethnic populations. Women with GDM are at risk for developing pregnancy complications [1,2]. Limited data regarding GDM suggest that hepatic metabolism may be significantly correlated with GDM. It is uncertain whether the elevated blood glucose in pregnant women is correlated with the parameters of kidney and liver function. The influence of GDM on both kidney and liver function-related variables has not been well characterized and is thus poorly understood.
Increased levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), and γ-glutamyl transferase (γ-GT) are associated with the incidence of type 2 diabetes [3,4]. It has been reported that the increased γ-GT level is an independent risk factor for GDM and may identify women as being at high-risk for the diagnosis of GDM [5,6]. Tests of hepatic function include assessments of the levels of albumin, bilirubin, enzymes, pre-albumin, etc. These parameters are routinely detected in diabetic clinics, but the parameters within normal range do not receive attention from clinicians. Increased plasma levels of cystatin C (Cys-C) were independently associated with preeclampsia [7], but not with GDM [8]. It should be stressed that previous studies only provided limited information and did not characterize the correlation with fetal outcome [5,6].
Pre-albumin, a marker for protein malnutrition, has been advocated as a useful marker for predicting diabetic complications. Our previous study showed that liver and kidney function parameters deteriorated, especially in diabetic ketoacidosis. Lower pre-albumin levels significantly reflected the presence of hyperglycemic emergencies [9]. To our knowledge, there is no available study on the patterns or prognostic value of pre-albumin levels in patients with GDM. Of these kidney and liver molecules, it is uncertain which one has the equal value as γ-GT in determining the prognosis GDM. Accordingly, we aimed to evaluate the changes in pre-albumin and other marker levels and their diagnostic value in reflecting the presence of GDM and fetal outcome. In particular, this study addressed whether the altered parameters were correlated with pregnancy complications.
Materials and Methods
Patient Recruitment and Exclusion Criteria
The study was performed at a medical college–affiliated hospital from January 2012 to October 2013. In the present study, the pregnant women who had not been previously diagnosed with diabetes were screened for GDM using a universal two-step GDM screening program as previously described [10]. The study was approved by the ethics committee of the hospital. Written informed consent was obtained from all of the participants.
Seventy-six consecutive women with GDM and 76 pregnancies with normal glucose tolerance (NGT) were included. The participants underwent a routine medical examination. Other inclusion criteria included age ≥18 y and singleton pregnancy. The GDM and control groups sustained a stable clinical status without inflammation or ischemia. The subjects with signs of any other pregnancy-associated disease were excluded. None of the participants had heart failure, hematologic disease, liver or kidney dysfunction, or fatty livers. GDM women may switch to multiple daily injections with human insulin or diet therapy. No oral antihyperglycemic medication was provided. The outcome was followed up until delivery. Deliveries were performed either vaginally or by elective cesarean section.
Evaluated Parameters
Blood samples were drawn at 7:30–8:00 in the morning after an overnight fast of 10 h. Blood samples were taken at baseline. Laboratory tests including ALT, AST, BChE, Cys-C, γ-GT, and pre-albumin were performed using routine laboratory methods [6,9]. Blood samples were collected into tubes for tests and immediately processed using an Olympus AU 2700 autoanalyser (Olympus, Tokyo, Japan). The glycated hemoglobin A1c (HbA1c) level was measured using Bio-Rad Laboratories Ltd. (Shanghai, China).
Statistical Analyses
The results are expressed as the mean ± SD for quantitative variables with normal distributions. Skewed parameters are presented as the median and range (min–max). The comparisons between the two groups were performed using the Student t-test. The chi-squared test was utilized for the comparison of other clinical features. Correlations between the observed variables were analysed by Pearson’s correlation test. The risk markers for the diagnosis of GDM were assessed by multiple logistic regression analyses. Receiver operating characteristic (ROC) curve analyses were performed to determine the diagnostic performance of each variable. Statistical analyses were conducted using SPSS 18.0 software (SPSS Inc., Chicago, IL) and MedCalc® version 12.1.4.0. A two-tailed p value < 0.05 was considered statistically significant.
Results
Clinical Characteristics and Biomarkers
As shown in [Table/Fig-1], a total of 152 pregnant women (NGT, n= 76; GDM, n= 76) were included in the study. Maternal weight, systolic blood pressure, plasma glucose, HbA1c, γ-GT, BChE, Cys-C, and pre-albumin were significantly higher in the GDM group than in the NGT group (p < 0.05 or p < 0.001). With respect to maternal age, serum ALT levels, and serum AST levels, there were no significant differences between the two groups at diagnosis.
Clinical and biochemical characteristics of the study participants
| Variables | NGT (n=76) | GDM (n=76) | p |
|---|
| Maternal age (years) | 28±5 | 29±5 | 0.136 |
| Gestational age at screening (weeks) | 24.63±0.49 | 24.58±0.57 | 0.542 |
| Maternal weight (Kg) | 64.54±4.01 | 76.03±3.64 | <0.001 |
| Systolic blood pressure (mmHg) | 126 ± 21 | 133 ± 11 | <0.001 |
| Diastolic blood pressure (mmHg) | 82 ± 10 | 81 ±16 | 0.254 |
| Fasting plasma glucose (mmol/L) | 4.36±0.30 | 5.29±0.29 | <0.001 |
| 2 hour postprandial glucose (mmol/L) | 7.83±1.20 | 10.47±2.2 | <0.001 |
| Glycated hemoglobin A1c (%) | 4.37±1.21 | 6.21±1.09 | <0.001 |
| Alanine aminotransferase (U/L) | 22.39±13.83 | 24.25±13.84 | 0.410 |
| Aspartate aminotransferase (U/L) | 20.75±6.51 | 22.43±8.19 | 0.163 |
| γ-glutamyl transpeptidase (U/L) | 12.92±6.00 | 18.04±7.86 | <0.001 |
| Butyrylcholinesterase activity (U/L) | 6142±1434 | 6704±1380 | 0.015 |
| Total bilirubin (γmol/L) | 7.54±3.29 | 7.01±2.73 | 0.281 |
| Direct bilirubin (γmol/L) | 3.02±1.33 | 3.02±1.44 | 0.983 |
| Total protein (g/L) | 66.21±3.54 | 67.24±4.37 | 0.113 |
| Albumin (g/L) | 37.16±2.86 | 38.71±3.89 | 0.003 |
| Globulin (g/L) | 29.05±3.22 | 28.54±3.14 | 0.322 |
| Pre-albumin (mg/L) | 230.87±44.20 | 248.14±44.92 | 0.018 |
| Cystatin C | 0.51±0.15 | 0.57±0.20 | 0.037 |
| Serum creatinine (γmol/L) | 42.64±9.16 | 45.08±9.72 | 0.114 |
| Uric acid (γmol/L) | 213.44±48.34 | 227.02±58.13 | 0.120 |
Correlation and Regression Analyses
In the GDM group, the serum levels of BChE activity was significantly correlated with fasting plasma glucose levels (r = 0.331, p = 0.004), Cys-C levels (r = -0.328, p = 0.004), and γ-GT levels (r = 0.266, p = 0.020). In the NGT group, the serum levels of BChE activity were significantly correlated with γ-GT (r = 0.300, p = 0.009). Other significant correlations were not observed.
With regard to delivery details, maternal and neonatal complications are shown in [Table/Fig-2]. The mean gestational age at delivery was significantly lower in the GDM than in the NGT group (p < 0.001). However, the rate of macrosomia was 17.1% (p = 0.010), and the newborn birth weights were significantly heavier in the GDM than in the NGT group (p < 0.001). There were significant differences between the two groups in terms of anomalies, preterm delivery, and Apgar scores at 1 and 5 min (all p < 0.05).
Maternal and fetal outcome
| Variables | NGT (n = 76) | GDM (n = 76) | p |
|---|
| Maternal | | | |
| Gestational age at delivery (weeks) | 39.55 ± 1.47 | 36.01 ±1.17 | <0.001 |
| Premature delivery | 6 (7.89%) | 14 (18.42%) | 0.020 |
| Pre-eclampsia | 1 | 2 | 0.380 |
| Postpartum hemorrhage | 0 | 0 | / |
| Fetal | | | |
| Newborn birth weight (grams) | 3378 ± 234 | 3701 ± 344 | <0.001 |
| > 4 kg | 2 (2.63%) | 13 (17.11%) | 0.010 |
| Anomalies | 0 | 2 | <0.001 |
| Neonatal death | 0 | 0 | / |
| Apgar at 1 min | 9.89 | 9.09 | <0.001 |
| Apgar at 5 min | 9.94 | 9.82 | 0.020 |
In terms of the presence of GDM, multiple logistic regression analyses with the four clinical variables (γ-GT, BChE, Cys-C, and pre-albumin) were performed. The serum levels of γ-GT and pre-albumin could act as risk markers for GDM (OR = 1.111, p = 0.001, 95% confidence interval 1.046–1.180; OR = 1.116, p = 0.010, 95% confidence interval 1.026–1.213, respectively). The second multiple logistic regression analyses for pregnancy complications with the three clinical variables (GDM status, γ-GT, and pre-albumin) were performed. Only the GDM status was a risk factor for pregnancy complications (OR = 1.405, p = 0.021, 95% confidence interval 1.140–1.996). Neither γ-GT nor pre-albumin could show predictive value for pregnant complications.
ROC Analyses
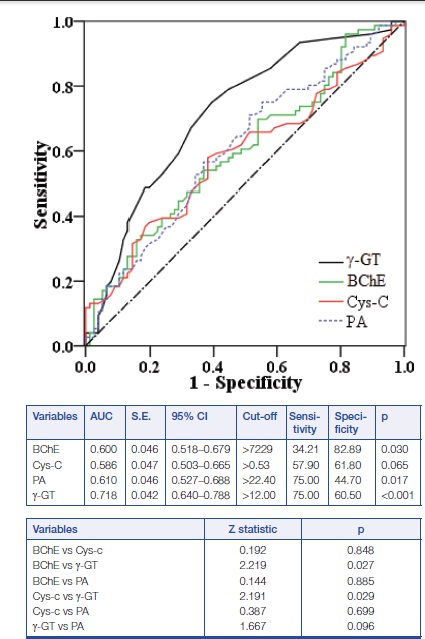
The ROC curves for γ-GT, BChE, Cys-C, and pre-albumin for NGT and GDM comparison are shown in [Table/Fig-3]. The AUCs were calculated for evaluation and compared. The levels of γ-GT, BChE, and pre-albumin showed significant differences, but no differences were found in Cys-C. To examine the specific ability of the levels of γ-GT, BChE, and pre-albumin in estimating the presence of GDM, the differences in the AUCs of the three markers were compared. The significant differences were observed and γ-GT showed significant advantage over BChE and pre-albumin factors (p < 0.05). No statistical significance was observed regard to the AUC between γ-GT and PA (z-value = 1.667, p = 0.096). PA was on the equal verge in reflecting the presence of GDM.
Comparison of the ROC plots of BChE, Cys-C, pre-albumin, and γ-GT between GDM patients and NGT controls. No difference was found between γ-GT and pre-albumin
Discussion
The present study demonstrates that the serum levels of albumin, BChE activity, γ-GT, Cys-C, and pre-albumin were mildly elevated in patients with GDM compared to NGT pregnant women. The biomarkers of kidney and liver function showed similar rising tendencies. Compared with γ-GT, the AUC of PA had no diagnosis difference. It is suggested that levels of PA had the equal value in reflecting the presence of GDM. Monitoring the status of these parameters in GDM, while perhaps not novel, could be characterized and understood to some extent.
It was observed that oxidative stress and inflammatory mediators were increased in patients with GDM [11,12]. The risk factors caused an impairment of renal excretion and hepatic metabolism. Previous studies confirmed that the increase in γ-GT level is an independent risk factor for GDM and identified women as high-risk for diagnosis of GDM [6]. In the present study, the diagnostic analysis presented here showed that both γ-GT and PA demonstrated ability in reflecting the presence of GDM. However, none of other markers were found to reflect pregnancy complications and fetal outcomes. Abnormal levels of glucose are associated with pregnancy complications [1,2].
As expected, only GDM status was found to be a risk factor for pregnancy complications. Although in the present study no variable was associated with newborn birth weight or other complications, further longitudinal, population-based studies are needed to explore the value of γ-GT and pre-albumin in GDM. Three pregnant women developed preeclampsia (one patient from control group, two patients from GDM). The mean levels of systolic blood pressure in GDM group were higher than in controls. GDM status was found to be a risk factor for pregnancy complications [13].
Plasma glucose was positively associated with levels of BChE, Cys-C, and γ-GT. Based on the present results and previous studies [5,6,9], it is suggested that hyperglycemia was the important cause of higher levels of BChE, γ-GT, Cys-C, and pre-albumin in women with GDM. Lowering blood glucose can reduce insulin resistance and oxidative stress and improve endothelial function in GDM [11]. Insulin therapy is the cornerstone of treatment for GDM, which can also result in an anti-inflammatory effect [11]. Our previous study demonstrated that insulin administration had a favorable effect on glucose levels, oxidative stress, and inflammatory cytokines [9,12]. Although the exact mechanism behind this phenomenon is unclear, this study showed that the most significant factor influencing γ-GT and pre-albumin levels should be diabetes status.
Pregnancy is susceptible to oxidative stress, and antioxidant defenses can be altered in response to elevated levels of oxidative stress. Glycemic levels in GDM were correlated with concentrations of lipid peroxides and increased protein oxidation [14]. Increased biomarkers of oxygen radical damage have been identified in GDM, such as myeloperoxidase and malonaldehyde levels [15,16]. These preliminary results demonstrated that the increase in the levels of BChE, γ-GT, Cys-C, and pre-albumin may be indicative of oxidative stress in GDM. GDM was found to be an independent risk factor for developing preeclampsia or infant risk [17]. Previous studies and our results suggested that GDM may promote a wide range of pathophysiological adaptations and enzymatic changes. The alterations observed in this study may be useful to help in understanding, at least in part, the pathophysiology of GDM.
Maternal overweight, excessive weight gain, and elevated blood glucose are common in the Chinese population. These metabolic risk factors were significantly associated with macrosomia and the low average age at birth. They should be controlled under the recommendations for pregnant population [18–20]. Accordingly, it can be concluded that the rate of macrosomia was increased in GDM. Further studies examining the rate of macrosomia in GDM are necessary.
As seen in our study, BchE, Cys-C, γ-GT were significantly elevated in GDM indicating their susceptibility, while serum ALT, AST, and creatinine did not change. An assessment of these surrogate markers of the kidney and liver will be helpful in the early monitoring of GDM patients. All these conditions are commonly found in GDM patients. One limitation of this study is that the results represent preliminary evidence from a small study of GDM patients with mild hyperglycemia. It is necessary for us to take future study.
Conclusion
In summary, elevated levels of BChE, γ-GT, Cys-C, and pre-albumin were shown to offer complementary information regarding GDM. GDM can result in further deterioration of the kidney and liver function variables. Both γ-GT and pre-albumin are suited for the monitoring of GDM.