Acne vulgaris, an extremely common skin condition, affects virtually all individuals at least once during their life. The incidence of acne is maximum at 18 y of age, but large number of men and women aged 20-40 y are also affected by the disorder [1]. It often leads to negative psychological consequences such as diminished self-esteem, depression and social withdrawal [2]. It affects the areas of the skin with the densest population of sebaceous follicles such as the face, upper part of chest, and back and is a result of interplay of four basic pathogenic events: increased sebum production, obstruction of pilosebaceous units by abnormal keratinization, inflammation, and proliferation of Propionibacterium acnes [3].
Acne vulgaris can be effectively managed by medical treatment. Management by topical tretinoin, a vitamin A derivative, is the primary treatment option for most forms of acne vulgaris [4] and has been the mainstay for comedolytic therapy for the last 25 y [5]. Tretinoin interacts with a family of nuclear retinoic acid receptors and reverses the process of abnormal follicular keratinization. It modulates the proliferation and differentiation of epidermal cells within the stratum corneum and restores desquamation of follicular cells, thus reducing the microcomedone formation and thereby non-inflammatory lesions [6,7]. It also exhibits anti-inflammatory effect through inhibition of toll like receptors thus reducing the inflammatory acne lesions as well [8].
Topical tretinoin has shown to be effective as a single agent therapy in patients with non-inflammatory comedones or mild-moderate inflammatory lesions [5], but is often associated with local adverse events such as erythema, dryness, peeling, and burning. These adverse events lead to lack of compliance and often necessitate discontinuation of treatment in some patients [9]. Moreover, low water solubility and instability of tretinoin in the presence of air, light and heat requires application of higher doses of the drug, further aggravating the adverse events. It has been shown that tretinoin induced irritation is dose and vehicle based [10] and various formulations of tretinoin like microspheres [11], incorporation of polymers [9,12] or nanogel [13] have been designed to reduce local adverse events.
Tretinoin nanogel, a novel formulation of tretinoin, has been developed using nanotechnology to overcome the shortcomings of its conventional formulation. This nanogel has been made using oil in water emulsion, with the size of oil droplets in the range of less than 0. 2 microns. Tretinoin being a lipophilic drug is dissolved in oil phase of this nanoemulsion, thereby increasing its penetration into the pilo-sebaceous glands due to the nano size of the droplets. Moreover, this nanogel formulation is designed to have good physical stability and uses aqueous-based gel vehicle with moisturizing properties to enhance its tolerability.
The present study was conducted to compare the efficacy and safety of this nanogel formulation of tretinoin (0.025% w/w) with conventional tretinoin formulation (0.025% w/w) in patients suffering from acne vulgaris of the face.
Materials and Methods
This prospective, randomized, open label, active controlled, parallel group, multicentre, post-marketing, clinical study was carried out at 10 centres geographically distributed across the country from April 2011 to September 2011. The study was conducted by qualified Dermatologists in compliance with the Indian Good Clinical Practice Guidelines and the ethical principles of Declaration of Helsinki. Study related activities were started after review and approval of the study by an Independent Ethics Committee (IEC) at each of the ten participating study centres and written informed consent of the patients.
Patients
Patients of either sex, 18 to 65 y of age, with acne vulgaris of face were enrolled in the study. Female patients were required not to be pregnant or lactating at the time of enrolment and not planning pregnancy during the study period. Patients with skin conditions of the face such as open or incompletely healed wounds at the affected site or acute eczemas, rosacea, perioral dermatitis, atopic / seborrheic dermatitis, or psoriasis, were excluded from the study. Patients with known hypersensitivity to preparations containing tretinoin, or any other related class of the compounds were also not eligible for enrolment in the study. Patients with significant cardiovascular, hepatic, renal or any other systemic illnesses could also not participate in the study. Patients who might have received any investigational medication in the previous three months or with continuing history of alcohol and/or drug abuse were not eligible for enrolment.
Patients were not permitted to take any other systemic or topical treatment for acne vulgaris concomitantly along with the study medication. Concomitant use of peeling agents, abrasive cleansers, strong drying agents, astringents or irritant products (aromatic and alcoholic agents) was not permitted as they produce irritant effects. Concomitant use of comedogenic cosmetics that can exacerbate acne lesions was also not permitted.
Study procedures
Patients satisfying the eligibility criteria were randomized as per a centralized computer generated randomization schedule to receive treatment with tretinoin nanogel formulation, 0.025% w/w (Nioret® Nanogel™, Cadila Healthcare Ltd, India) or a marketed tretinoin conventional gel formulation, 0.025% w/w (Retino-A® Gel, Johnson & Johnson, India) in a 1:1 ratio. Patients were instructed to wash the entire face each night with a non-abrasive cleanser, rinse immediately, pat dry the face and apply a thin film of the study medication to the acne affected areas using the fingertips, avoiding the eyes and lips. Patients were advised to avoid exposure to the sunlight by using a sunscreen of SPF 30 and/or wearing protective clothing. The total study duration was 12 wk and patients were followed-up on outpatient basis at an interval of 4 wk after the initiation of therapy.
Efficacy of the medication was assessed by counting the number of inflammatory (papules, pustules, nodules & cysts), non-inflammatory (open & closed comedones) and total lesions at each visit and grading the severity as per the acne severity score [14,15] [Table/Fig-1]. The investigators’ were also asked to rate the global assessment of efficacy at the end of the study on a four-point rating scale wherein “Excellent” was total or near total resolution of acne lesions; “Good” was significant resolution of acne lesions; “Fair” was some resolution of acne lesions and “Poor” was no resolution or worsening of acne lesions compared to baseline.
Acne severity grades [14,15]
| Grade | Score | Description |
|---|
| Clear | 0 | Normal-appearing, clear skin with no evidence of acne vulgaris |
| Almost clear | 1 | Rare non-inflammatory lesions present, with rare non-inflamed papules (papules must be resolving and may be hyperpigmented, although not pink-red) |
| Mild | 2 | Some non-inflammatory lesions are present, with few inflammatory lesions (papules/pustules only; no nodulocystic lesions) |
| Moderate | 3 | Non-inflammatory lesions predominate, with multiple inflammatory lesions evident: several to many comedones and papules/pustules, and there may or may not be one small nodulocystic lesion |
| Severe | 4 | Inflammatory lesions are more apparent, many comedones and papules/pustules, there may or may not be a few nodulocystic lesions |
| Very severe | 5 | Highly inflammatory lesions predominate, variable number of comedones, many papules/pustules and many nodulocystic lesions |
Tolerability of study medication was assessed by documenting adverse events on each of the scheduled visits, with details such as date of onset and end (duration), severity (mild, moderate or severe), its treatment and final outcome. Patients were asked to report any intolerable adverse events to the investigator at the earliest via telephone or personal visit. The relationship of the respective study medication to each of the adverse event was evaluated by the World Health Organization-Uppsala Monitoring Centre (WHO-UMC) criteria. The severity of adverse events were assessed by the investigator’s clinical judgment using predefined criteria wherein “mild” was an event that was easily tolerated by the patient, causing minimal discomfort and not interfering with everyday activities; “moderate” was an event that was sufficiently discomforting but did not interfere with the normal everyday activities; and “severe” was an event that was discomforting enough to interfere with the normal everyday activities. The investigators’ rated the global assessment of tolerability at the end of the study on a four-point rating scale (Excellent - no adverse event reported; Good - mild adverse event(s) reported which subsided with or without medication and did not necessitate stoppage of study medication; Fair - moderate to severe adverse event(s) reported which subsided with or without medication and did not necessitate stoppage of study medication; Poor - severe or serious adverse event(s) which necessitated stoppage of study medication).
Statistical Analysis
The primary efficacy variable in the study was the percentage change in the number of inflammatory lesions, non-inflammatory lesions and total lesions at the end of the therapy. The secondary efficacy variables were success rate defined as the percentage of patients rated as having “clear” or “almost clear” grades of acne severity score at the end of the study degree of improvement in the acne severity score, and the investigators’ global assessment of efficacy.
Efficacy and safety assessments were carried out in the Intention-To-Treat (ITT) population which included all those patients who presented for the first follow up visit after randomization and had properly filled CRFs. The missing data values were completed by last observation carried forward (LOCF) procedure. Efficacy data of continuous variables are presented as mean, standard deviation (SD), & 95% confidence intervals (CI) and for ordinal/nominal variables as frequency (number) and percentage of patients along with 95% CIs. Student’s t-test, Chi-square Test and Fischer’s Exact Test were applied for statistical analysis by two-tailed assessments, according to the data characteristics. p- values < 0.05 were considered as statistically significant.
Sample size was based on data (SD=33.9) from previous studies [16]. It was estimated that a minimum of 83 patients in each group would be required to establish superiority of the nanogel formulation to the conventional gel formulation of Tretinoin in terms of percentage reduction of total lesions at .05 significance level, with at least 90% power and a superiority margin of 5 %. Considering a dropout rate of 20%, a total of 208 patients (104 in each group) were planned for the study.
Results
A total of 207 patients with acne vulgaris of the face were enrolled in the study at ten different centres across India. One hundred and nine (109) out of these 207 patients were randomized to receive tretinoin nanogel formulation (TNG group); while 98 patients received treatment with tretinoin conventional gel formulation (TCG group). A total of 189 patients in the study qualified for the intention to treat efficacy analysis (99 in TNG group and 90 in TCG group) while 202 patients were considered for safety analysis (105 in TNG group and 97 in TCG group). [Table/Fig-2] shows the flow of the patients enrolled in the study.
Flow of the patients enrolled in the study
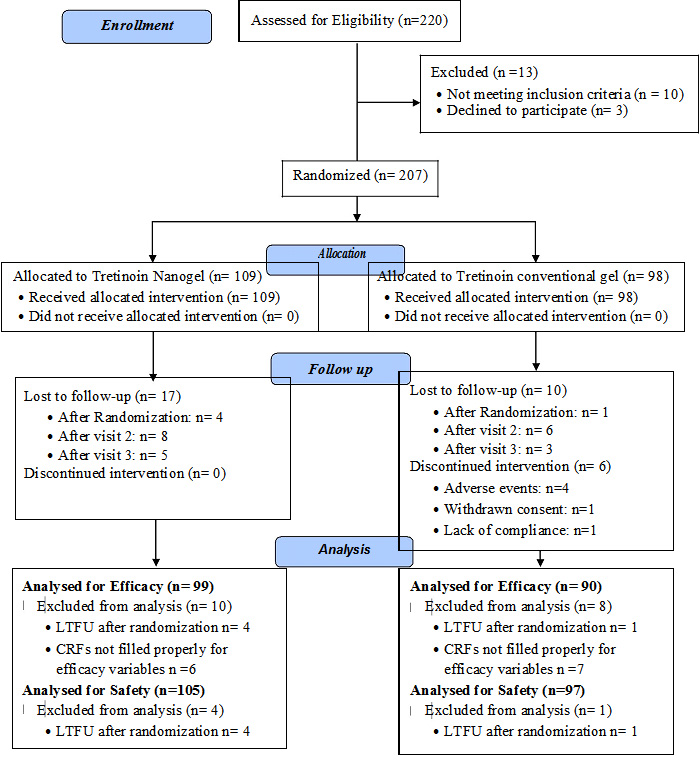
Demographics & Baseline characteristics: Patients enrolled in the TNG and the TCG group had similar demographic profiles. [Table/Fig-3] shows the details of the demographic profile of the patients enrolled in the study and [Table/Fig-4] shows the baseline disease characteristics of the patients considered for the intention to treat efficacy analysis. The severity of acne at the baseline was similar in both the treatment groups as reflected by the similar number of total, inflammatory, and non-inflammatory lesions as well as acne severity grades.
Demographic characteristics of the patients enrolled in the study [Mean ± SD/No.(%)]
| Characteristics | TNG group (n=109) | TCG group (n=98) | p-value (Significance) |
|---|
| Age (yr) | 21.9 ± 4.1 | 23.0 ± 5.0 | 0.07 (NS) |
| sex | Males | 60 (55.0%) | 52 (53.1%) | 0.77 (NS) |
| Females | 49 (45.0%) | 46 (46.9%) |
| Height (cm) | 162.3 ± 9.1 | 160.0 ± 8.4 | 0.07 (NS) |
| Weight (kg) | 56.4 ± 8.6 | 57.2 ± 10.5 | 0.35 (NS) |
| Duration of illness (months) | 26.5 ± 29.9 | 31.6 ± 39.0 | 0.35 (NS) |
TNG = Tretinoin Nanogel, TCG = Tretinoin Conventional Gel, SD = Standard Deviation, NS = Non-significant
Baseline disease characteristics of the patients considered for intention to treat efficacy analysis [Mean ± SD/No.(%)]
| Characteristics | TNG group (n=99) | TCG group (n=90) | p-value (Significance) |
|---|
| No. of Lesions | Inflammatory | 13.9 ± 11.5 | 12.7 ± 9.0 | 0.46 (NS) |
| Non-Inflammatory | 18.8 ± 13.6 | 18.8 ± 11.7 | 0.99 (NS) |
| Total | 32.6 ± 20.2 | 31.3 ± 17.0 | 0.62 (NS) |
| Acne Severity | Grade 0 | 0 (0%) | 0 (0%) | 0.28 (NS) |
| Grade 1 | 0 (0%) | 0 (0%) |
| Grade 2 | 6 (6.1%) | 11 (12.2%) |
| Grade 3 | 64 (64.6%) | 48 (53.4%) |
| Grade 4 | 27 (27.3%) | 28 (31.1%) |
| Grade 5 | 2 (2.0%) | 3 (3.3%) |
TNG = Tretinoin Nanogel, TCG = Tretinoin Conventional Gel, SD = Standard Deviation, NS = Non-significant
Efficacy assessments: Both the inflammatory and non-inflammatory acne lesions reduced after the initiation of therapy in all the enrolled patients in both the treatment groups during the course of the study. Reduction in the number of the inflammatory lesions was significantly (p = 0.02) greater in the TNG group (78.1 ± 25.2%) as compared to the TCG group (66.9 ± 35.2%) while the non-inflammatory lesions showed a trend towards greater reduction with the nanogel as compared to the conventional gel (p = 0.1) [Table/Fig-5].
Mean per cent reductions in acne lesions in both the study groups at week 12 as compared to the baseline (Mean ± SD)
| Characteristics | TNG group (n=99) | TCG group (n=90) | p-value (Significance) |
|---|
| Inflammatory Lesions | 78.1 ± 25.2% | 66.9 ± 35.2% | 0.02 (S) |
| Non-inflammatory Lesions | 68.6 ± 22.6% | 63.0 ± 23.5% | 0.10 (NS) |
| Total Lesions | 72.9 ± 21.2% | 65.0 ± 26.9% | 0.03 (S) |
TNG = Tretinoin Nanogel, TCG = Tretinoin Conventional Gel, SD = Standard Deviation, S=significant, NS= not-significant
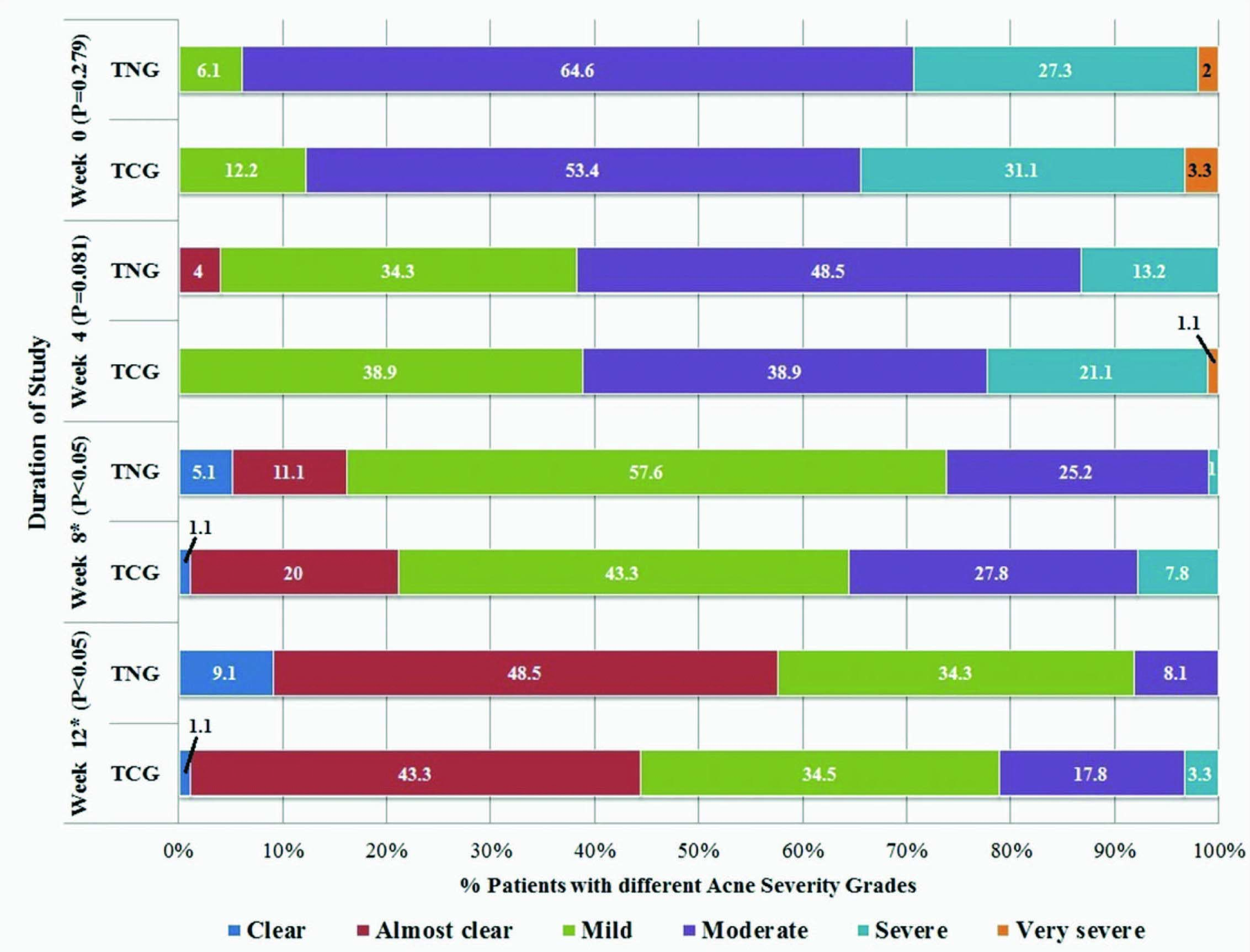
Based on the change in the acne severity grades, 57.6% (47.7-66.8%) patients in the TNG group achieved ‘treatment success’ as compared to 44.4% (34.6-54.7%) patients in the TCG group (p = 0.07). Significantly greater number of patients (p = 0.01) achieved grade 0 i.e., “complete” clearance of acne lesions in the TNG group (9.0%; 4.9-16.4%) as compared to the TCG group (1.1%; 0.2-6.0%). The change in the acne severity grades reported in each of the treatment groups during the course of the study is shown in [Table/Fig-6]. Significantly greater number of patients (p = 0.01) had at least a two grade change in their acne severity at the end of the treatment in the TNG group (66.6%; 53.8-72.4%) as compared to the patients in the TCG group (45.6%; 35.6-55.8%). Further, none of the patients in any of the groups showed an increase in acne severity grades while five (5.0%) patients in the TNG group and six (6.6%) patients in the TCG group had the same acne severity grades at the end of the study as compared to the baseline.
Percentage of patients with different acne severity grades during the study
TNG = Tretinoin nanogel, TCG = Tretinoin conventional gel, TNG group: n=99, TCG group: n=90
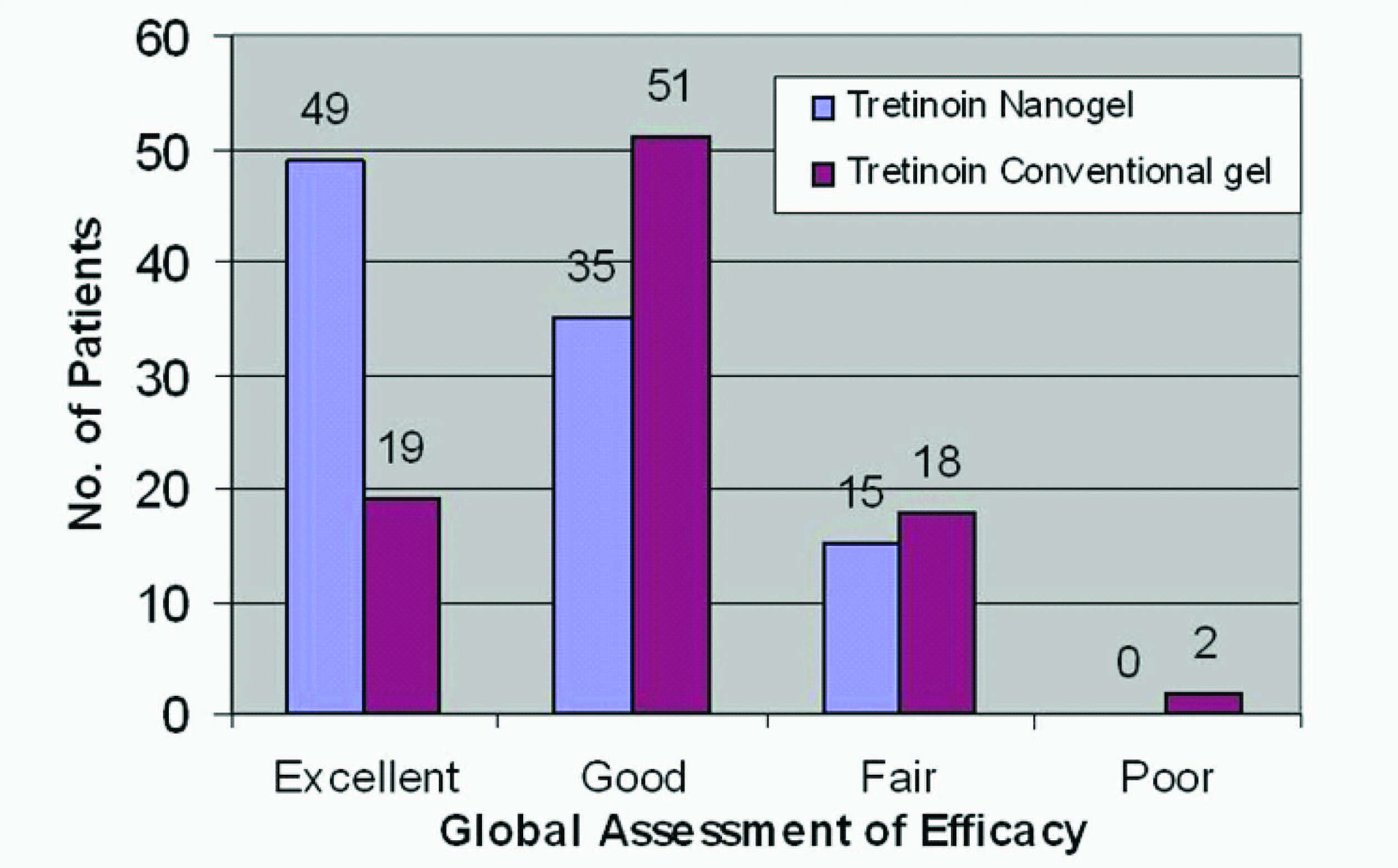
Tretinoin nanogel was shown to be significantly better (p < 0.01) on investigator’s assessment of global efficacy of study medication [Table/Fig-7]. [Table/Fig-8] shows the change in acne lesions as observed at baseline and at the end of treatment in a selected patient from the TNG group.
Global assessment of efficacy
The effect of Tretinoin nanogel on acne lesions after 12 weeks of treatment (A & B - baseline and C & D - after 12 weeks of treatment with Tretinoin nanogel)

Tolerability assessments: Local adverse events were seen in both the study groups but the patient adverse event rate was significantly less (p = 0.04) in the TNG group as compared to the TCG group. A total of 14 adverse events were reported by 14 patients (13.3%) in the TNG group while 28 adverse events were reported in 24 patients (24.7%) in the TCG group. Dryness was the most common adverse event reported in both the treatment groups (4.8% in the TNG group and 8.2% in the TCG group) while adverse events such as peeling of skin, burning sensation and photosensitivity were reported in patients using the conventional gel only [Table/Fig-9].
Adverse event profile of patients in the study
TNG = Tretinoin nanogel, TCG = Tretinoin conventional gel, TNG group: n=105, TCG group: n=97
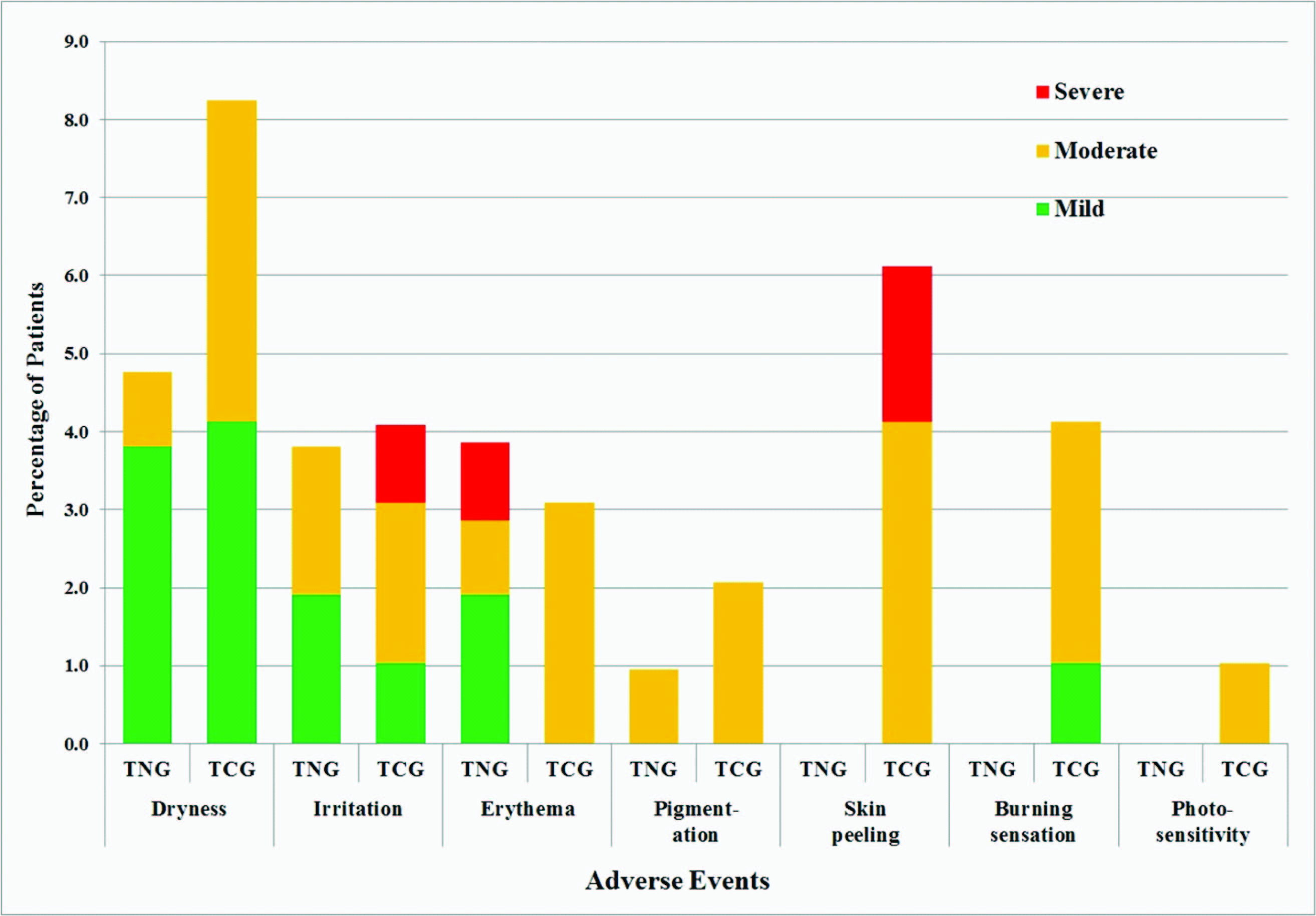
Severity of adverse events was “mild” in majority of the patients (eight out of 14 cases) treated with the TNG while it was “moderate” in majority of patients treated with the TCG (19 out of 28 cases). Only one patient in the TNG group had “severe” erythema while three patients in the TCG group had severe reactions (two had peeling of skin and one had irritation). None of the patients enrolled in the TNG group discontinued the study medication due to any adverse event during the entire course of study while four patients using TCG discontinued the study due to adverse events; two of these patients had “severe” skin peeling while one patient had erythema & irritation of “moderate” intensity and one patient had burning sensation of “moderate” intensity. All the adverse events in both the treatment groups had a “possible” association with the respective study medication and resolved with/without symptomatic treatment during the course of the study.
Global assessment of tolerability also showed that tretinoin nanogel was significantly better tolerated as compared to the conventional gel (p = 0.003). In the TNG group, 91 (86.7%) patients were rated to have an “excellent” tolerability to the study medication, eight patients (7.6%) were rated as having a “good” tolerability, six patients (5.7%) were rated as having a “fair” tolerability and none of the patients was rated to have a “poor” tolerability while in the TCG group only 73 (75.3 %) patients were rated to have an “excellent” tolerability, six patients (6.2%) were rated to have a “good” tolerability, 14 patients (14.4%) were rated to have a “fair” tolerability and four patients (4.1%) were rated to have a “poor” tolerability to the study medication.
Discussion
Topical tretinoin has been the foundation of acne therapy and has been found to be highly effective as monotherapy and when used in combination with other systemic and topical agents [5]. It is primarily a comedolytic agent with good activity against inflammatory lesions as well. Its main disadvantage has been the cutaneous adverse events reported with its use. A new nanogel formulation of tretinoin has been developed to further improve its efficacy and tolerability. The present randomized, multicentric study evaluated this novel nanogel formulation of tretinoin with the conventional gel formulation in patients with acne vulgaris of the face in the Indian population. The present study intended to assess the therapy with topical tretinoin for a period of 12 wk during which optimum treatment response is generally obtained. Moreover, monotherapy with tretinoin was investigated to determine the comparative clinical effectiveness as well as safety of the nanogel formulation in the absence of the other confounding treatment variables.
Evaluation of the mean percent change in the lesion count in this clinical study indicated that the nanogel formulation of tretinoin is significantly better in reducing the inflammatory and the total lesion count as compared to the conventional tretinoin gel formulation. It also showed a trend towards better efficacy in reducing the number of non-inflammatory lesions. The mean reduction in the lesion counts recorded in our study with the nanogel formulation ranged from 68-78% while that with the conventional formulation ranged from 63-67%. The results with the conventional gel formulation in our study are comparable to the various other large multicentric studies carried out in various set-ups with tretinoin conventional gel. Results from an Indian [17] double blind study evaluating effects of different formulations of Tretinoin in treatment of acne over period of three months have shown a 62% reduction in number of lesions while another study from Europe [18] has shown a reduction of around 60% in the number of both the inflammatory and the non-inflammatory lesions.
The change in acne severity grades and global efficacy rated by the investigators also showed superiority of the nanogel formulation as compared to the conventional formulation. More than 66% patients in the tretinoin nanogel group had at least a two grade change in their acne severity at the end of treatment as compared to 45% patients in the conventional gel group. Similarly, 49 patients (49.5 %) in the TNG group were rated to have an “excellent” efficacy, while only 19 (21.1%) patients in the TCG group were rated the same.
The enhanced efficacy of the nanogel formulation as compared to the conventional formulation is attributed to the advanced nanogel technology which allows increased concentration of the active drug in the pilosebaceous unit [13,19]. Nanogels have shown to improve the dermal localization of the various topical therapeutic agents including tretinoin [20] by increasing the surface area of the drugs and thereby increasing their solubility and permeation through tissue barrier [21,22]. Tretinoin in the nanogel formulation is transported to the pilosebaceous unit in an encapsulated form (encapsulated in the nanogel matrix) probably via the trans-follicular route [13,19]. Previous studies with the nanogel formulations have also shown to have an antimicrobial activity against P. acnes, one of the major bacterium involved in the pathogenesis of acne, similar to that of the topical 2% erythromycin products [23]. The enhanced antibacterial activity of the nanogel results from the fusion of the discrete droplets of oil with the bacterial cell walls or viral envelopes, leading to destabilization and disruption of the organism’s lipid envelope [24]. This enhanced anti-inflammatory action of the nanogel formulation has led to a significantly greater reduction in the number of the inflammatory lesions as compared to the conventional formulation in this study.
Another reason for improved efficacy of the nanogel formulation of tretinoin is the enhanced photo-stability of tretinoin in the novel formulation as compared to the conventional gel formulation [13]. Tretinoin is highly susceptible to sunlight and a large amount of locally applied tretinoin degrades on the skin surface within 1–2 h of application [25]. Previous reports have shown that 81% of tretinoin in the conventional formulation is degraded after 120 min of exposure to simulated solar UV irradiation [26] while another study showed that 71% of tretinoin in the nanogel formulation remains in the active form even after 180 minutes of irradiation [13]. This enhanced photo-stability of the novel formulation, due to better adsorption and embedment of tretinoin into the nanoparticle matrix [13], increases the concentration of the active drug at the site of application and thus improves its efficacy.
Safety evaluation of study medications in this clinical trial showed adverse events such as dryness, irritation, erythema, pigmentation, peeling of skin and photosensitivity. Although the adverse event profile was similar to that seen in the other clinical trials [17,18] and was consistent with the internationally published literature [27]. The incidence and severity of these adverse events was lower in patients treated with the novel nanogel formulation as compared to the conventional gel formulation of tretinoin. This better tolerability profile of the novel tretinoin formulation is also attributable to the nanogel technology. The reduced incidence of skin irritation is hypothesized to be the result of encapsulation of tretinoin in the nanogel matrix which reduces the contact of the acidic function (-COOH) of tretinoin (the triggering factor for topical adverse events) with the stratum corneum [28]. Further, better moisturizing properties of the nanogel formulation also lead to favourable local tolerance.
One of the major limitations of the study was its open label design. Due to the different physical appearance of the tested formulations, a double blind study could have been possible only by using complex study design like the double dummy technique. This would have led to additional topical application of vehicle base affecting the treatment response, adverse event profile and patient compliance.
Conclusion
The results of this clinical trial for the treatment of acne vulgaris of the face suggest that tretinoin 0.025% nanogel formulation is more efficacious and better tolerated than its conventional formulation. Future studies with more robust study designs such as double blind, double dummy technique or split face comparisons assessing monotherapy and combination therapy with other agents such as antimicrobials or benzoyl peroxide for extended study durations of longer than 12 wk can further extend the therapeutic role of this novel formulation in the management of acne vulgaris.
Acknowledgements
Authors would like to thank Dr. Ravindra Mittal, Dr. Amit Kubawat and Mr. Prafulla Pawar, all from Department of New Product Development, Cadila Healthcare Ltd. Ahmedabad for their active contribution in design and conduct of the study. Authors would also like to thank Dr. Shafiq Sheikh (Department of New Drug Delivery Systems, Cadila Healthcare Ltd. Ahmedabad) for development of this novel formulation.
Contribution of Each Author and Guarantors
BC, MA, MR, PV, RA, SS, ST, SA, SP and AS were involved in concept, design & conduct of clinical study and manuscript review. JS was involved in data management, statistical analysis, manuscript preparation, manuscript editing and manuscript review. The study was sponsored by Cadila Healthcare Ltd., Ahmedabad and the Sponsor i.e. Cadila Healthcare Ltd. Ahmedabad is the Guarantor of the data specified.
TNG = Tretinoin Nanogel, TCG = Tretinoin Conventional Gel, SD = Standard Deviation, NS = Non-significant
TNG = Tretinoin Nanogel, TCG = Tretinoin Conventional Gel, SD = Standard Deviation, NS = Non-significant
TNG = Tretinoin Nanogel, TCG = Tretinoin Conventional Gel, SD = Standard Deviation, S=significant, NS= not-significant