A Rare Collision Tumour of Infiltrating Ductal Carcinoma and Squamous Cell Carcinoma of Skin Overlying Breast: A Case Report
RL Geetha1, R. Kalyani2, Murthy. V. Srinivas3, Chinnathambi Shakthidasan4
1 Assistant Professor, Department of Pathology, ESIC Medical College and PGIMSR and Model Hospital, Rajajinagar, Bangalore, Karnataka, India.
2 Associate Professor, Department of Pathology, ESIC Medical College and PGIMSR and Model Hospital, Rajajinagar, Bangalore, Karnataka, India.
3 Professor & Head of Department, Department of Pathology, ESIC Medical College and PGIMSR and Model Hospital, Rajajinagar, Bangalore, Karnataka, India.
4 Resident, Department of Pathology, ESIC Medical College and PGIMSR and Model Hospital, Rajajinagar, Bangalore, Karnataka, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Kalyani. R, Associate Professor, Department of Pathology, ESIC Medical College and PGIMSR, Bangalore, Karnataka, India. E-mail : drkalyanir@rediffmail.com
Collision tumour is the concrescence of two neighbouring independent neoplasm occurring in the same site. Collision tumours are rare and are reported in various organs. Collision tumours in breast are reported in various combinations. The treatment of these tumours is not tailored and prognosis depends on the histologic type and pathologic stage of the most aggressive component.
We report a rare case of breast collision tumour of infiltrating ductal carcinoma and squamous cell carcinoma of skin overlying breast in a 44-year-old female.
Axillary lymph nodes, Mammogram, Mastectomy
Case Report
A 44-year-old female presented with lump in the left breast since three months which was gradually increasing in size. Patient had pain and tenderness in the mass with no discharge from nipple. Personal and family history was not significant. With consent of the patient, local examination was done which revealed mass in the left breast in the upper outer quadrant and measured about 6x3x4cms. Ipsilateral axillary lymph nodes were palpable. A nodule was also noted in the skin about 5 cms lateral to the nipple measured 1 cm across [Table/Fig-1]. Provisional clinical diagnosis of carcinoma of breast probably with a satellite nodule and axillary lymph node deposits was made. Fine needle aspiration cytology (FNAC) of the breast mass revealed high cell yield consisting of round tumour cells having hyperchromatic nucleus, increased N:C ratio, coarse granular chromatin, some with prominent nucleoli and moderate amount of eosinophilic cytoplasm. These cells were arranged in loosely cohesive sheets, ductular pattern and in discretes. Background showed hemorrhage and necrosis. A FNAC diagnosis of infiltrating ductal carcinoma was offered. The biochemical and hematological laboratory investigations were within normal limits. Ultrasound and Mammogram of left breast showed a well defined mass of 10 cms across. Computed tomography showed a mass in left breast with features highly suggestive of malignant lesion. Chest X-ray and ultrasound examination of abdomen were within normal limits suggesting no metastatic deposits. The patient received three cycles of neoadjuvant chemotherapy with Cyclophosphamide, Adriamycin and 5 Fluorouracil following which there was decrease in the size of the mass (from 10 cms across to 6x4x3 cms). Whole body CT was done which did not show any metastatic deposits. Patient under-went modified radical mastectomy of left breast with ipsilateral axillary lymph nodes dissection.
Gross photograph showing resected breast with cutaneous nodule (arrow)
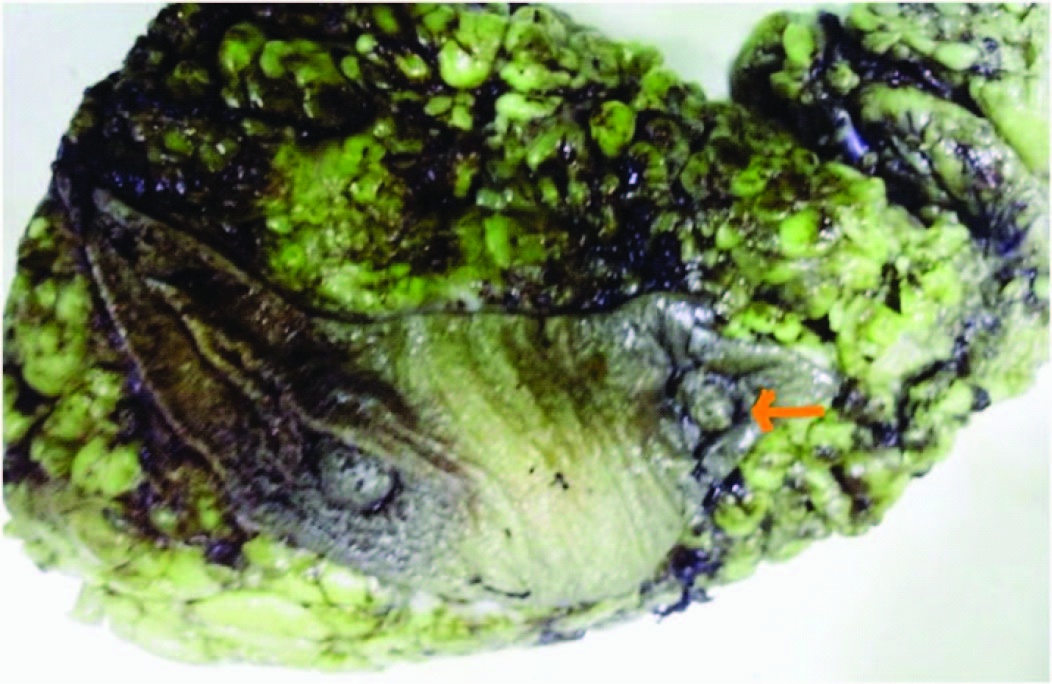
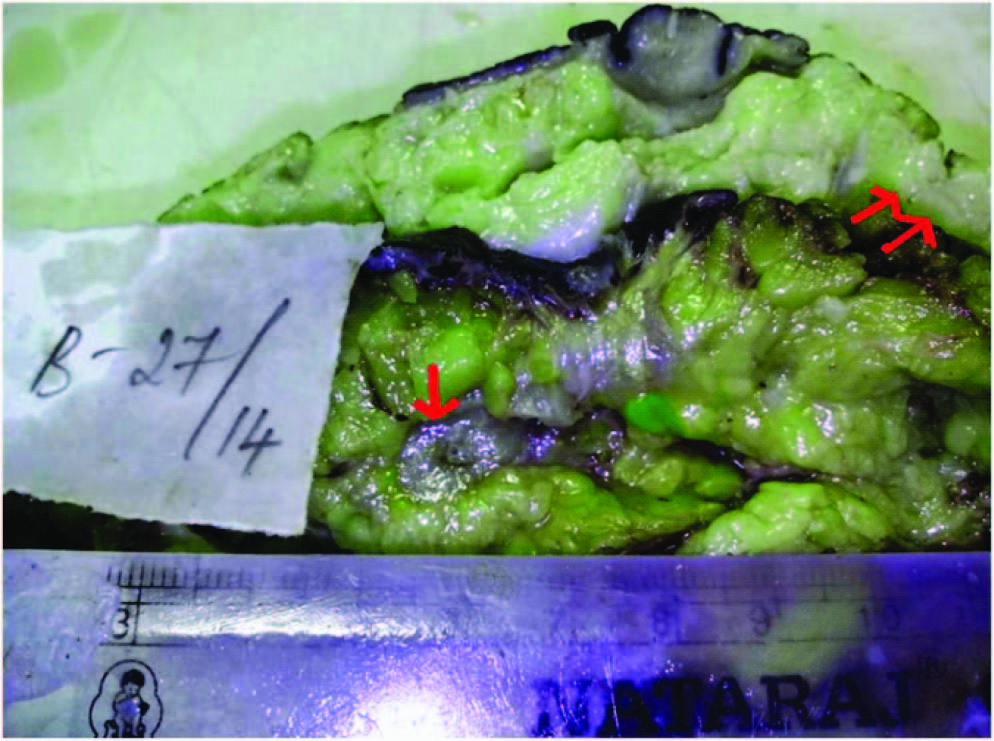
Gross Examination of the resected specimen measured 20x16x7 cms, showed an elliptical shaped skin with normal nipple and areola. Adjacent cutaneous nodule measured 1x1 cms. On cut section, a firm, grey white breast mass in the upper outer quadrant measuring 6x4x3cms was noted. Cut section of adjacent cutaneous nodule was grey white [Table/Fig-2]. The axillary tail showed 17 greyish white firm lymph nodes, the largest measured 1 cm across and smallest 0.5cms, cut section was grey white and homogenous. Representative bits from breast mass, adjacent cutaneous nodule, resected margins and lymph nodes were given for histopathological examination.
Gross photograph showing the cut section of breast mass (single arrow) and nodular area (double arrow)
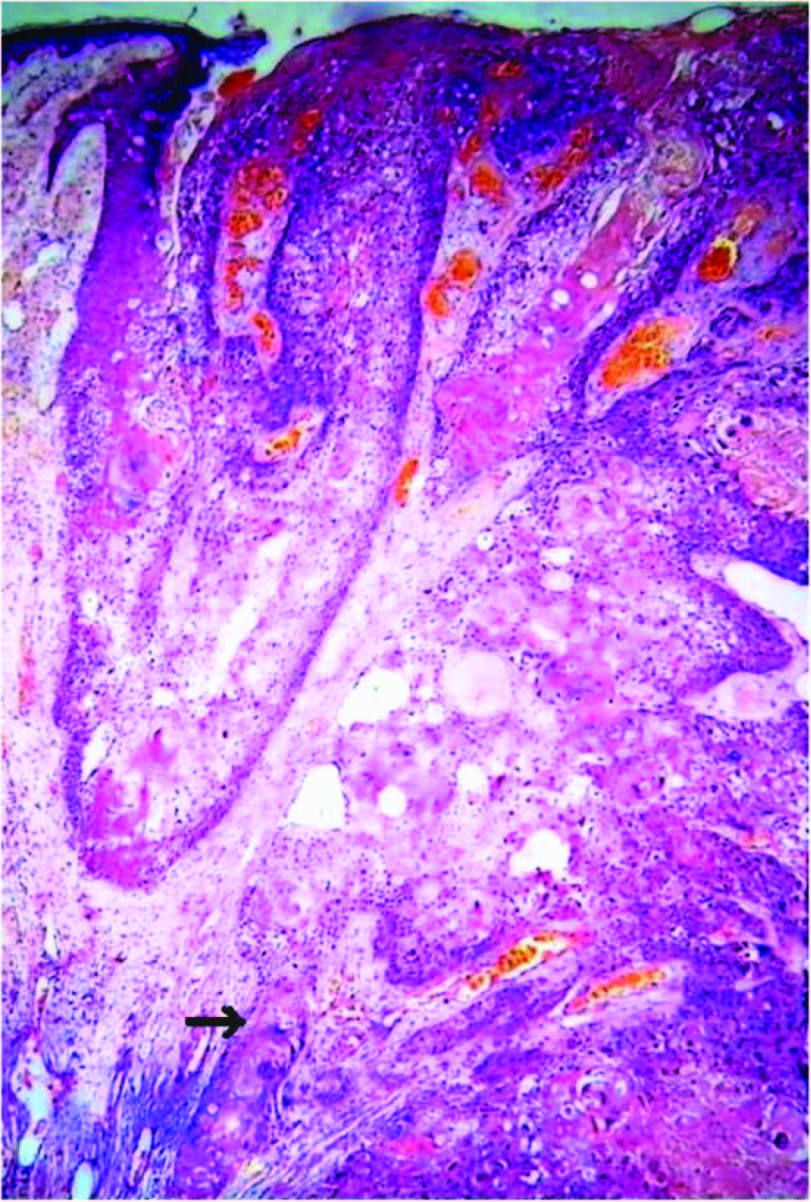
Microscopy of breast mass showed epithelial tumour cells having marked anisonucleosis, moderate amount of eosinophilic cytoplasm arranged in nests, tubules and in sheets infiltrating the stroma [Table/Fig-3]. Sections from adjacent cutaneous nodule interestingly showed sheets of polyhedral epithelial tumour cells extending from skin and infiltrating into deeper subepithelial stroma having bizarre nuclei, moderate to abundant amount of cytoplasm exhibiting keratin differentiation at places forming keratin pearls [Table/Fig-4]. There was no histological evidence of transition from IDC to SCC. A histopathological diagnosis of infiltrating ductal carcinoma of the breast NOS (not otherwise specified) with cutaneous SCC was made. Sections from axillary lymph nodes showed metastatic deposits of only squamous cell carcinoma. The surgical margins were free from the tumour.
Microphotograph showing round tumour cells in ductular pattern infiltrating the stroma, features of infiltrating ductal carcinoma of the breast. H&E x100

Microphotograph showing polygonal tumour cells extending from the squamous epithelium infiltrating into stroma with keratin pearls (arrow), features of infiltrating squamous cell carcinoma of skin over the breast H&E x100
Immunohistochemistry of IDC for oestrogen (ER), progesterone (PR) and HER2 Neu receptors revealed ER, PR negative and HER2 Neu (human epidermal growth factor receptor 2) positive [Table/Fig-5]. All the three receptors were negative in squamous cell carcinoma component.
Microphotograph showing Her2neu positive in infiltrating ductal carcinoma component of breast carcinoma. HER2neuX100

Postoperative recovery was uneventful. Patient took six cycles of chemotherapy with no recurrences. Later patient was lost for follow-up.
Discussion
Breast cancer is the second most common cancer in the world and the most frequent cancer among the women with an estimated 1.67 million new cancer cases diagnosed in 2012 (25% of all female cancers) [1]. Invasive ductal carcinoma (IDC) is the commonest histologic variant of carcinoma breast and about 75% of the diagnosed IDC are defined as IDC – NOS [2–4].
Squamous cell carcinoma (SCC) is the commonest cancer of the skin. It occurs in other organs like oral cavity, larynx, pharynx, esophagus, stomach, anus, urinary bladder, vagina, cervix and lung. It is rarely encountered in the skin overlying the breast, which has to be distinguished from primary breast or metastatic squamous cell carcinoma from elsewhere in the body. SCC can occur as a complication of chronic inflammation [1].
Collision tumours are rare clinical entities in which two histological distinct tumour types show involvement in the same site. It can be defined as concrescence of two neighbouring independent neoplasm. The synonyms are composite or synchronous tumours. The occurrence of these tumours in the breast is extremely rare. Collision tumours are reported in breast; however, the incidence is not recorded because of its rarity and many published articles are case reports and case series. Majority of breast collision tumours are reported are in fourth to sixth decade of life and majority are seen in females [1,5,6]. In our case the patient is a female in fifth decade.
Majority of reported breast collision tumours are synchronous in presentation. Some of the reported collision tumours of breast are primary SCC and invasive lobular carcinoma, metaplastic SCC with invasive lobular carcinoma, inflammatory breast carcinoma and malignant phylloides tumour, IDC and breast MALT lymphoma, chronic lymphocytic leukemia and lactating adenoma, IDC and B cell lymphoma [Table/Fig-6] [1,5,6]. While cutaneous SCC of the breast in association with IDC of the ipsilateral breast as in our case is very rare and on extensive literature search we were not able to find any such reports suggesting that this is an extremely rare type collision tumour of breast. In the present case the tumour had synchronous presentation.
Shows the reported collision tumour of breast in various studies
| Sl. No | Various studies | Age (Years) | Gender | Histologic type |
|---|
| 1 | Khan MI et al., [1] | 38 | Female | Metaplastic squamous cell carcinoma with invasive lobular carcinoma |
| 2 | Sentani K et al., [5] | 57 | Female | Primary SCC and invasive lobular carcinoma |
| 3 | Shin YD et al., [6] | 45 | Female | Inflammatory breast carcinoma and malignant phylloides tumour |
| 4 | Present case | 44 | Female | Infiltrating Ductal carcinoma and squamous cell carcinoma of skin over breast |
Microscopy of SCC in our case was similar to SCC elsewhere in the body with well formed keratin pearls and metastatic deposits in the axillary lymph nodes. The cutaneous SCC can be differentiated from primary SCC of breast by certain immunohistochemistry markers as; HMW-CK positive for both, primary breast SCC positive for CK 19, CK7, CK8 contrary to cutaneous SCC and negative for ER and PR [7]. Most of SCC are more sensitive to radiotherapy [1]. In our case, IDC was ER and PR negative but HER2neu positive and cutaneous SCC was negative for all three markers.
Collision tumours are reported in esophagus especially in cardioesophageal junction. Examples of collision tumours in esophagus are SCC and small cell carcinoma, adenocarcinoma and lymphoma, adenocarcinoma and small cell carcinoma, adenocarcinoma and SCC. Collision tumours are also reported in other organs [8,9].
The treatment of these rare collision tumours of breast are lacking standardization and tailored treatment. The prognosis of these collision tumours remains unclear; however depends on the histopathologic subtype and pathologic stage of the more aggressive tumour subtype in the breast. The SCC component in collision tumour usually grow rapidly, lymph node metastasis occur in approximately 54% of cases, give rise to more distant metastasis and has bad prognosis [5,7]. In our case, the patient had axillary lymph node metastasis of only SCC and not IDC indication the aggressive behavior of SCC component compared to IDC. However the patient was followed up for only six months without evidence of recurrence.
Conclusion
One should be aware that collision tumour occurs in breast and histologic typing / pathologic staging is important to standardize the treatment and predict the prognosis. The collision tumour of breast presented here i.e., IDC with SCC of skin over breast is very rare and as far as our knowledge goes this is the first of its kind in the English literature.
[1]. Khan MI, Haque AU, Younar M, Zafar A, Shah H, Metaplastic Squamous cell carcinoma in association with Invasive lobular breast carcinoma with metastasis to axillary lymph nodesJ Ayub Med Coll Abbotlabad 2011 23(4):135-37. [Google Scholar]
[2]. Singletary SE, Parekh LP, Bland KI, Treatment Trends in Early Stage Invasive Lobular carcinoma. A Report From the National Cancer Data BaseAnn Surg 2005 242(2):281-89. [Google Scholar]
[3]. Globocan 2008 update: Cancer Prevalence estimates are now available. Media centre. IARC News. 2011. www.iarc.fr/en/media-centre/iarcnews/2011/globocan2008-prev.php (23/12/2014) [Google Scholar]
[4]. Yerushalmi R, Hayes MM, Gelmon KA, Breast Carcinoma-rare types: review of literatureAnn oncol 2009 20(11):1763-70. [Google Scholar]
[5]. Sentani K, Tashiro T, Oue N, Yasuiw Synchronous Squamous cell carcinoma of the breast and Invasive lobular carcinomaAPMIS 2007 115:1422-25. [Google Scholar]
[6]. Shin YD, Lee SK, Kim KS, Park MJ, Kim JH, Yim HS, Collision tumour with inflammatory breast carcinoma and malignant phyllodes tumour: A case report and literature reviewWorld Journal of surgical oncology 2014 12:5 [Google Scholar]
[7]. Latha PS, Chaitanya B, Rajasekhar SR, Squamous cell carcinoma of the breast-A rare entityInternational Journal of Medical Research and Review 2013 1(3):134-37. [Google Scholar]
[8]. Jingpei Li, Xiaoke Chen, Yaxing Shen, Yingyong Hou, Shumin Zhang, Hao Wang, A rare collision tumour of squamous carcinoma and small cell carcinoma in esophagus involved with separate lymph nodes: a case reportJ Thorac Dis 2013 5(5):203-06. [Google Scholar]
[9]. Spagnolo DV, Heenan PJ, Collision carcinoma at the esophagogastric junction: report of two casesCancer 1980 46:2702-08. [Google Scholar]