The use of pesticides as agents of deliberate self-harm is on the rise, and more than 500,000 cases are reported annually [1]. The suicidal consumption of rodenticides is common in the Indian sub continent [2]. The composition of different rodenticides varies, and different rodenticides have different toxicities. However, many of them are hepatotoxic, and can cause Acute Liver Failure (ALF) [3]. In the absence of a definite antidote, mortality in patients admitted with rodenticide consumption is high [4]. N acetyl cysteine (NAC) is used in the management of ALF secondary to consumption of toxic doses of acetaminophen (paracetamol) [5]. Some studies show that NAC is also useful in the treatment of Non Acetaminophen induced ALF (NAI) [6]. Our study aimed to find whether NAC has a definite role in the management of rodenticide consumption.
Materials and Methods
Institution Ethics Committee approval was obtained for the study. Case sheets of all consecutive patients availing the inpatient services of a tertiary medical college hospital in a city on the West Coast of South India between January 2010 and December 2012 and admitted with an alleged history of rodenticide consumption were surveyed and data was extracted and analysed. The history of rodenticide consumption documented in the case sheet had been obtained from the patients and in some instances from the patients’ bystanders. Details of the dose and form of the poison, where available, were collected. Case records which showed that the patient had consumed more than one poison, or in which the poison consumed was not clearly mentioned to be rodenticide, were excluded. Patients were analysed with respect to age, sex and mode of presentation. Serial charting of Liver function tests (LFT) was done. In patients in whom NAC had been administered, the interval between consumption of rodenticide and starting NAC was noted. The outcome in patients treated with NAC was compared to outcomes in those not treated with NAC. The primary outcome was mortality due to ALF induced by rodenticide consumption, and the secondary outcome was the adverse effects of NAC when used in this situation.
Statistical Analysis
The SPSS software version 17 was used to analyse the results. Multivariate analysis was done. A p-value of <0.05 was considered significant.
Results
A total of 100 patients were admitted to the hospital during the study period with a history of ingestion of rodenticide. The sex ratio was equal, with half the patients being female.
Emesis was a common presenting symptom, with 67 patients presenting with vomiting. Only one of the hundred patients presented with altered sensorium [Table/Fig-1].
Groups were comparable with respect to sex
| NAC | Total |
|---|
| Yes | No |
|---|
| SEX | Male | Count | 13 | 37 | 50 |
| % | 50.0% | 50.0% | 50.0% |
| Female | Count | 13 | 37 | 50 |
| % | 50.0% | 50.0% | 50.0% |
| Total | | Count | 26 | 74 | 100 |
| | % | 100.0% | 100.0% | 100.0% |
The mean admission ALT/AST was 306/451, discharge ALT/AST was 291/302, and peak ALT/AST was 451/655. The median and interquartile ranges of admission AST in those who died and those who were discharged were respectively 28/1182 and 30/47. We found that patients who had received NAC had lower mortality. However, they had higher initial AST/ALT values probably due to the tendency of the treating physician to start NAC only when there was significant enzyme elevation in the absence of clear guidelines on its management. Patients who received NAC had mean admission AST/ALT of 1186/681, compared to those who did not receive NAC (468/370).
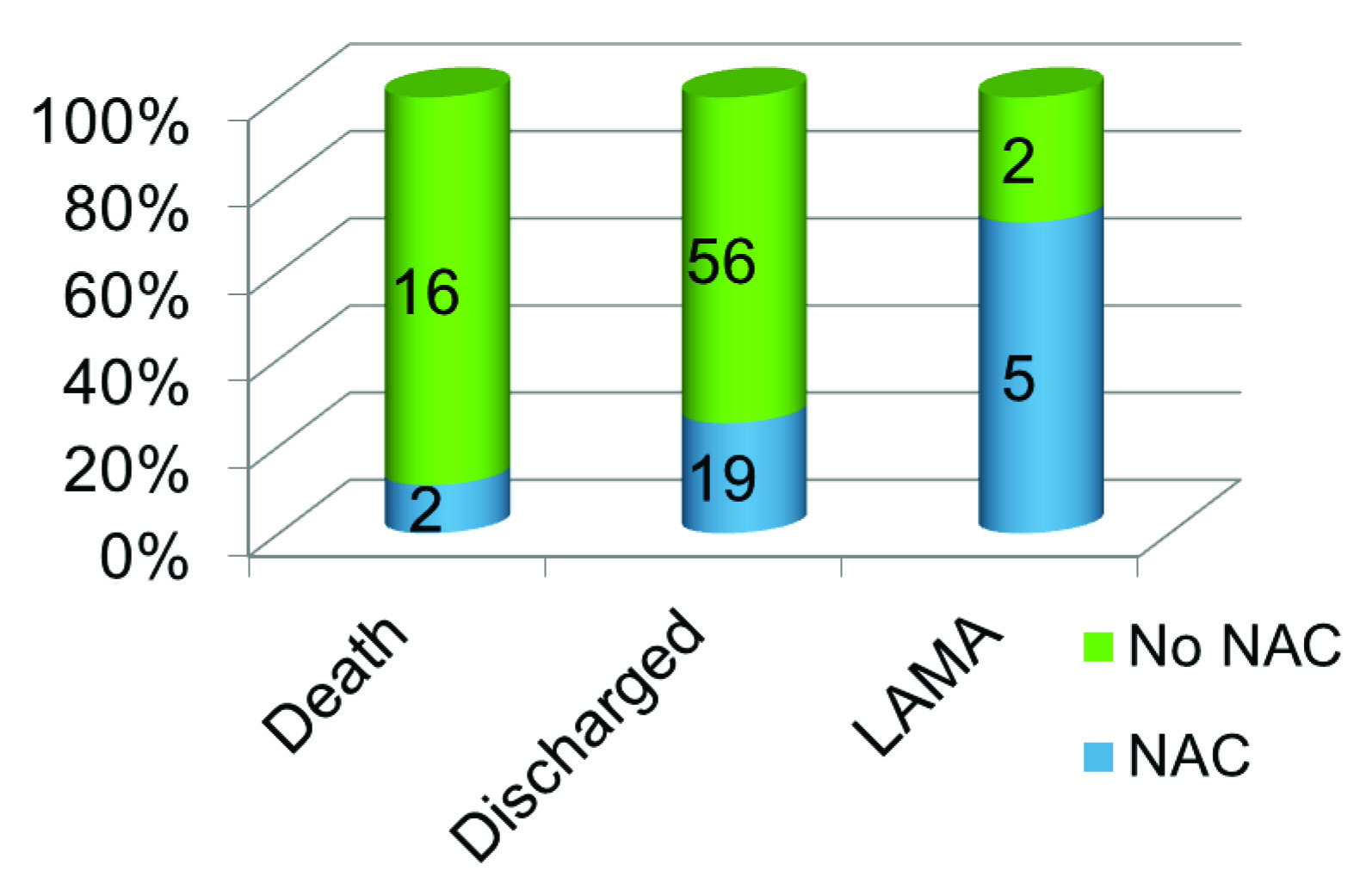
The overall mortality of patients admitted with rodenticide consumption in our centre during the study period was 18%. Twenty six patients out of 100 were treated with NAC. Out of these 19 (73.1%) recovered, 2 (7.7%) died and 5(19.2%) were discharged against medical advice. Of the 74 who were not treated with NAC, 16 (21.6%) died, and 56 (75.7%) recovered. The difference between patients who had received NAC and had not received NAC was highly significant. The two patients who had received NAC and expired had a greater time lag to the administration of the drug.
Discussion
The number of cases of pesticide poisoning is on the increase globally, with around 300,000 cases being reported every year [7]. Rodenticides are composed of superwarfarins, thallium, barium carbonate, aluminum phosphide (AlP) and zinc phosphide. They are inexpensive and highly toxic pesticides [8]. They are easily available in the Indian subcontinent and are common agents in deliberate self-harm, especially among the agricultural community. The high fatality due to ALF induced by rodenticides makes the need for an antidote urgent and imperative.
Rodenticides are toxic to the liver and heart. Rodenticides containing aluminum or zinc phosphide are hepatotoxic and can cause acute fulminant hepatic failure (ALF). Patients admitted with aluminum or zinc phosphide poisoning often develop hepatic necrosis, renal failure, metabolic acidosis and refractory hypotension [9]. In the absence of any known antidote for AlP poisoning, ALF in the setting of rodenticide poison consumption is often fatal, with mortality rates ranging from 40 – 80% [10].
Aluminum phosphide (AlP) and zinc phosphide are the hepatotoxic forms of rodenticide. AlP is a solid fumigant and is available in the form of dark grey tablets. Commercial names include celphos, alphos, quickphos and phostoxin [11]. When ALP comes in to contact with moisture it liberates the highly toxic phosphine gas. Phosphine inhibits cellular oxygen utilization by its effect on mitochondria, and also by decreasing the activity of cytochrome oxidase [12]. It also liberates highly reactive hydroxyl radicals [13]. Reactive oxygen species toxicity is believed to be key in the genesis of AlP induced toxicity. Rodenticides also contain 2 – 5% of yellow phosphorus [14]. Phosphorus poisoning is also known to cause ALF [15].
Acute liver failure (ALF) is a rare and severe, life threatening medical emergency. It is defined as the rapid development of acute liver injury with impaired synthetic function (INR > 1.5) and encephalopathy in a person who previously had a normal liver [16]. Causes of ALF include infections, metabolic derangements like Reyes syndrome and acute fatty liver of pregnancy. Toxins like paracetamol, tetracycline, and Amanita phalloides can also cause ALF [17]. The clinical features of ALF include progressively worsening jaundice, fetor hepaticus and coma. Biochemical derangements include increased INR and elevated transaminases. Emergent recognition and management of ALF is crucial, as untreated, it has a poor prognosis. Only 25% of patients with ALF recover spontaneously [18]. Those who do not, often require a liver transplant, which might not always be possible in resource constrained settings.
NAC is used in the treatment of ALF secondary to acetaminophen toxicity. Toxic doses of acetaminophen deplete glutathione levels and inhibit cytosolic glutathione transferase activity. NAC exerts its action by acting as an intracellular precursor or substitute of glutathione [19]. Additionally, NAC has anti-inflammatory, inotropic and vasodilatory effects [20]. In ALF, oxygen supply and utilization at the cellular level are disrupted. This perhaps partly explains the beneficial effect of NAC, which has been shown to increase oxygen supply in the microcirculation [21].
Guidelines do not exist regarding routine use of NAC in non acetaminophen induced ALF, and in hepatic failure due to rodenticide consumption. Patients admitted with ALF after phosphorus ingestion Have sometimes been managed with NAC [22]. Case reports and small studies do show that NAC may be useful, especially in patients who are not candidates for liver transplantation. A placebo controlled trial where patients with non-acetaminophen induced ALF were treated with NAC showed a significant survival benefit in patients treated with NAC, especially those in the early stages of encephalopathy. However, studies in children with ALF treated with NAC show mixed results, with some studies showing a clear benefit [23]. However, in other studies, treatment with NAC had no therapeutic advantage [24].
At present, no definite antidote for aluminum phosphide poisoning has been identified. Various substances like boric acid [25], coconut oil [26], digoxin [27], and magnesium [28] have been proposed for treatment in rodenticide containing AlP poisoning, however the evidence for them, at best, remains tenuous.
In our study, all patients admitted with history of suicidal ingestion of rodenticide were given supportive care. Hematological and biochemical investigations were sent from the casualty, as soon as patient presented to hospital. Gastric lavage and skin decontamination were done before the patient was shifted to the intensive care unit. Patients received intravenous fluids (IVF), enteral or parenteral nutrition depending on the grade of encephalopathy, and proton pump inhibitors. Injectable vitamin K was administered if PT/INR was elevated. Antibiotics were given in case of suspected or documented infections, and fresh frozen plasma was given when increased INR co existed with active bleeding. Electrolytes and blood glucose were monitored and abnormalities, if present, were corrected. Wherever necessary, mechanical ventilation was initiated.
In the absence of any recognized antidote for rodenticide poisoning, the management remains supportive and empirical. Treatment methodologies differ between hospitals, and within a hospital between units. In view of the high mortality associated with rodenticide consumption, and the low cost and relatively benign adverse effect profile of NAC, and the absence of guidelines on management of rodenticide consumption, physicians in our center began to treat patients admitted with rodenticide consumption with NAC. In settings where there is no access to liver transplantation, AlP poisoning can result in mortality of up to 70% [29]. In the study period of three years, a total of 100 patients were enrolled in the study, out of which 30 were in 1st year, 33 were enrolled in 2nd year, and 32 in year 3rd. A total of 26 patients admitted with rodenticide consumption were treated with NAC, and these patients had been admitted and treated in the last eight months of the study period. There was no major change in ICU staffing/functioning or organizational character during this three year period that would have impacted patient outcome. NAC was given at the dose of 150mg/kg for one hour followed by 50 mg/kg over four hours, followed by 100mg/kg over 16 h.
In our center, cases admitted with rodenticide consumption were mainly from the agricultural community, and many of them were young. Rodenticide consumed in patients admitted to our center was mainly composed of aluminum phosphide, zinc phosphide and yellow phosphorus. Patients presented most commonly with symptoms of GI toxicity including vomiting and abdominal pain. This is similar to mode of presentation in other studies [10]. Elevation of liver enzymes occurred usually on the 2nd to 3rd day after admission. This is fairly typical with phosphorus poisoning [14], and it is important that patients continue to receive intensive monitoring and care duringand after the first three days following ingestion, even if overt signs of hepatotoxicity are absent. In our study, the liver was the main organ involved, and serum creatinine (at presentation) was normal in all patients studied. This differs from a study conducted in North India where 25% had elevated s. creatinine, and elevated values were found to correlate with mortality [30]. This discrepancy is perhaps because of different compositions of the pesticide that are available.
In our study the total mortality in patients admitted with rodenticide consumption was 18%. Patients who received NAC had a mortality rate of 7.7%, compared to 21.6% in those patients who did not receive NAC [Table/Fig-2]. A similar dramatic difference is seen in outcomes in studies where NAC has been used in treatment of Non acetaminophen induced ALF (73% without NAC, 53% with NAC) [6]. The difference between deaths in patients who had received only usual supportive care and in those who also received NAC was highly significant.
Outcomes with and without NAC
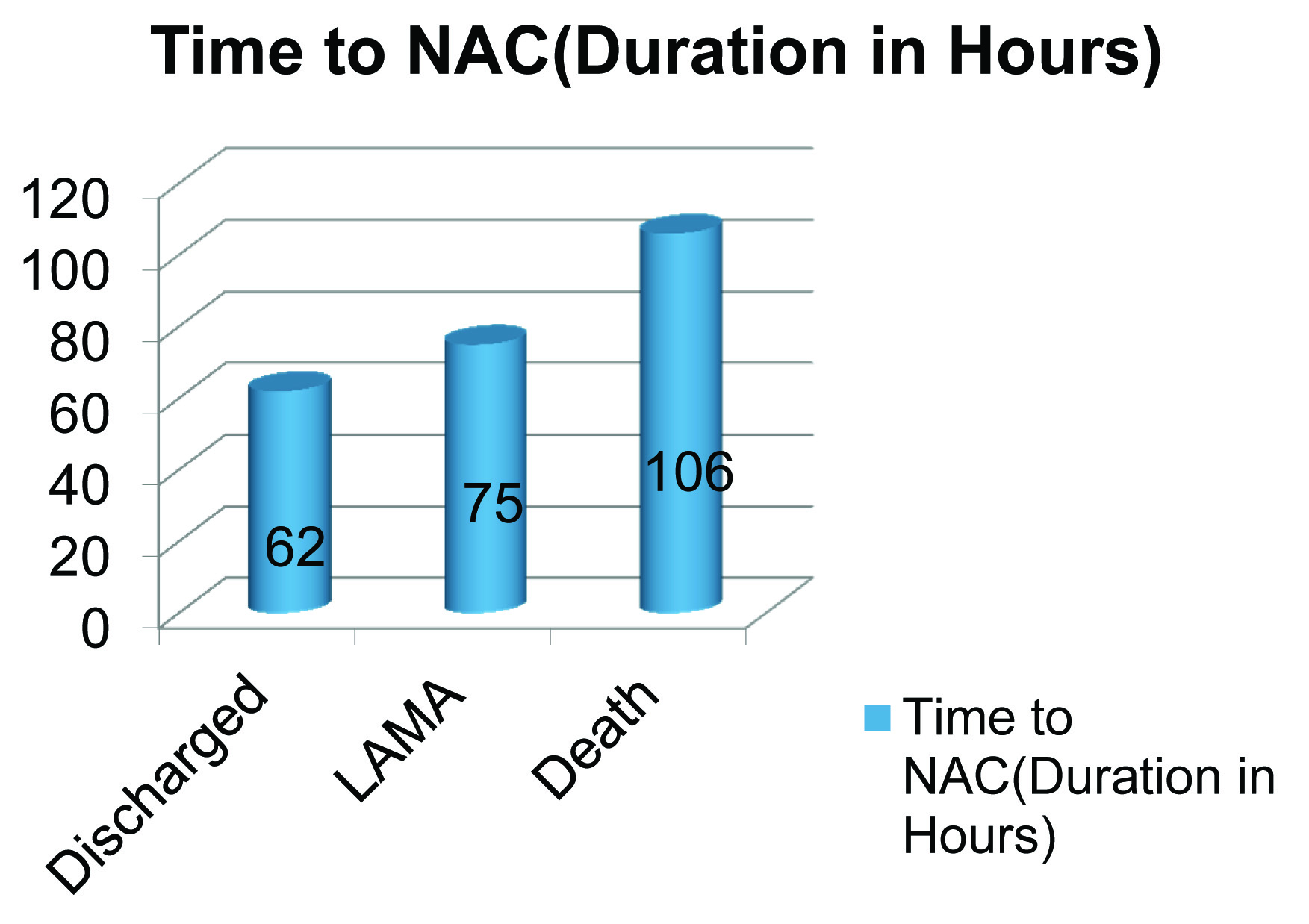
In our study all patients with significant elevations of liver enzymes who had not received NAC died [Table/Fig-3]. Of the patients who had elevated enzymes and had received NAC, the two deaths were seen in patients with a mean lag time between consumption and administration of 106 h, as opposed to 62 h for those who had received NAC and survived [Table/Fig-4] Patients who died had a higher elevation of AST/ALT and cause of death was ALF and GI bleed.
Outcomes in patients treated with and without NAC
| NAC | Total |
|---|
| Yes | No |
|---|
| Outcome | Death | Count | 2 | 16 | 18 |
| % | 7.7% | 21.6% | 18.0% |
| Home | Count | 19 | 56 | 75 |
| % | 73.1% | 75.7% | 75.0% |
| LAMA | Count | 5 | 2 | 7 |
| % | 19.2% | 2.7% | 7.0% |
| Total | | Count | 26 | 74 | 100 |
| | % | 100.0% | 100.0% | 100.0% |
LAMA: Left against medical advice
The two groups were comparable in almost all clinical, biochemical and demographic parameters [Table/Fig-1,5,6], however the admission values of AST/ALT were higher in the treatment group. This is perhaps explained by the fact that patients with higher elevations of liver enzymes were more likely to receive NAC, and patients with less severe elevation were less likely to have received NAC, especially in the initial part of the study period when evidence as to the beneficial effects of NAC was lacking.
Groups were comparable with respect to age
| Group Statistics |
|---|
| NAC | N | Mean age±Std. Deviation | T |
|---|
| AGE | Yes | 26 | 29.88±8.964 | .898 |
| No | 74 | 27.34±13.418 | p=.371 ns |
Groups were comparable with respect to history of alcohol abuse
| NAC | Total |
|---|
| Yes | No |
|---|
| Alcohol | Yes | Count | 6 | 23 | 29 |
| % | 23.1% | 31.1% | 29.0% |
| No | Count | 20 | 51 | 71 |
| % | 76.9% | 68.9% | 71.0% |
| Total | | Count | 26 | 74 | 100 |
| | % | 100.0% | 100.0% | 100.0% |
Limitations
This study was not a placebo-controlled trial. The authors accept that retrospective analyses using historical controls are subject to bias, and have limitations. However, after the initial few cases treated with NAC started showing better outcomes, it was considered unethical to withhold NAC from the others, as case fatality with rodenticide consumption and hepatic failure is extremely high. Therefore the controls were mainly historical. NAC was given to all patients, regardless of the delay between consumption and admission to hospital. Data of time interval between rodenticide consumption and hospital admission was not tabulated, which might be considered a limitation, as delayed hospital admission would imply delay in supportive measures such as gastric lavage.
Secondly, the composition of rodenticides, and amount consumed was not always available from the case sheet. The toxicity of rodenticides especially ALP depends on whether the tablet was exposed to the atmosphere or not. These details were not always available from the case sheets surveyed. The lack of data regarding composition of rodenticides is a further limiting factor in the study.
Conclusion
In spite of these limitations, we believe that these results have great clinical significance. Most patients admitted with history of suicidal consumption of rodenticide were young, and belonged to poorer socio-economic sections. Therefore, treatment with NAC, which is inexpensive and relatively safe, would be a viable treatment option for patients admitted with rodenticide consumption, Who develop ALF and are not eligible for liver transplant.
LAMA: Left against medical advice