Tuberculosis meningitis (TBM) is a serious public health problem in developing countries as it leads to significant mortality and residual neurological sequelae. The estimated mortality due to TBM in India is 1.5 per 100,000 population [1]. Despite being an endemic country for TB, data regarding clinical, radiological and laboratory (biochemical and microbiological) parameters and final outcome of adult TBM patients is sparse in India. Analysis of such variables in various countries has shown association of various factors with the prognosis of the disease like age, stage of disease, level of consciousness, presence of extraneural TB, isolation of Mycobacterium tuberculosis from CSF, biochemical studies and hydrocephalus [2–4]. The availability of such data in a high burdened developing country like India may help in prediction of the prognosis of such patients and help in early intervention of preventive measures to improve the subsequent outcome of the patients. The present work was planned as a prospective study to assess the clinical profile, laboratory values and neuroimaging features of adult TBM patients and to find out predictors of mortality in these patients so as to frame a model in future which will help in predicting the prognosis of the patients.
Materials and Methods
This prospective study was conducted over seven months from August 2010 to February 2011 in Department of Medical Microbiology and Neurology of our tertiary care referral centre in North India. The study was approved by Institute Ethics Committee and was conducted after taking informed consent from the patients. A total of 55 cases of age >12 y diagnosed as TBM based on clinical criteria were included in the study and were divided into confirmed and suspected group according to criteria as given by Ahuja et al., [5]. All these cases fulfilled Thwaites’ index (score of 4 or less suggests TBM) [6]. Patients were also clinically divided into 3 stages using Medical Research Council staging system [7]. The control group included 60 patients with differential diagnosis of TBM in which 30 cases of meningitis like bacterial other than Mycobacterium tuberculosis (11), viral (15) and fungal (4) meningitis and rest 30 included patients of non-infectious illness of central nervous system as described previously. Detailed clinical history including age and sex was taken emphasising on presence of fever, headache, vomiting, altered sensorium in a prescribed proforma. Patients were clinically evaluated for signs of meningeal irritation, raised intracranial pressure, cranial nerve deficits, focal neurological deficits and evidence of TB elsewhere in the body. Laboratory parameters included CSF cell count and levels of protein, glucose and adenosine deaminase (ADA). CSF evaluation for Mycobacterium tuberculosis was done by multiplex PCR using two primers, i.e. IS6110 and MPB64. The radiological details of contrast enhanced CT brain, MRI brain, CT chest, CT abdomen were also noted. The response to treatment was judged by gain of weight, appetite and improvement of behaviour. The cases were treated with four drug regimen of antitubercular drugs according to the national programme in India.
Statistical Analysis
Statistical analysis was done using SPSS 15.0 for Windows. Tests used included Student’s t-test, Fischer’s exact test and Chi square test. Univariate and multivariate analysis was done to find out the predictors of mortality. Odds ratio was used to denote the risk of various factors. p-value of <0.05 was considered to be significant.
Results
The 55 TBM cases were divided into confirmed (n=9) and suspected (n=46) group. The suspected cases were further classified into highly probable (n=4), probable (n=39) and possible (n=3) cases. Four patients were HIV positive (7.27%) and belonged to the confirmed TBM group.
Age and Gender
Among 55 patients, 61.8% patients were males and 38.2% were females. 45.5% of the patients were of the age group between 21-40 years while 21.8% and 32.7% patients belonged to age group <=20 y and >= 40 y respectively. The mean age of TBM patients and control group was 36.42±16.20 y and 40.88±17.57 y respectively.
Signs & Symptoms, Sites of Involvement and Comorbidities
The duration of symptoms in all TBM cases was > 14 d (mean of 16 d). The most common symptoms included fever (90.9%), headache (72.7%), neck rigidity (67.3%), altered sensorium (65.5%) and vomiting (54.5%). The most commonly involved cranial nerve was 6th nerve (9.1%). The symptoms of fever, headache, vomiting, seizures, anorexia and neck rigidity were found to be significantly high in TBM patients (p<0.05) [Table/Fig-1]. The most common presentation of extraneural TB was miliary distribution (16%) followed by pulmonary involvement, lymphadenitis and abdominal TB.
Clinical features and CSF characteristics of study subjects
| TBM patients | Control group | P value |
|---|
| Clinical features |
| Fever | 50 (90.9%) | 41(68.3%) | 0.003 |
| Headache | 40 (72.7%) | 28(46.7%) | 0.008 |
| Vomiting | 30 (54.5%) | 21(35.0%) | 0.040 |
| Seizures | 13(23.6%) | 14(23.3%) | 1.000 |
| Anorexia | 31(56.4%) | 4(6.7%) | <0.001 |
| Coma | 10(18.18) | 4(6.66%) | 0.0860 |
| Altered sensorium | 36 (65.5%) | 34(56.7%) | 0.347 |
| Neck rigidity | 37 (67.3%) | 19(31.7%) | <0.001 |
| Cranial nerve palsy | 8(14.5%) | 3(5.0%) | 0.114 |
| 6th nerve | 5 (9.1%) | 0 (0%) | 0.023 |
| 7th | 4 (7.3%) | 2 (3.33%) | 0.424 |
| 3rd nerve | 1 (1.8%) | 1(1.7%) | 1.000 |
| 5th | 1 (1.8%) | 0(0%) | 0.478 |
| Hemiparesis/ Hemiplegia | 8 (14.5%) | 7(11.66%) | 0.783 |
| Evidence of extra neural tuberculosis | 16 (29.1%) | 0(0%) | <0.001 |
| History of TB | 9(16.4%) | 0(0%) | <0.001 |
| CSF characteristics |
| Protein >100 mg% | 47(85.45%) | 18(30%) | <0.001 |
| CSF TLC >20 cells/mm3 | 46(83.63%) | 18(30%) | <0.001 |
| CSF sugar <2/3 CBS | 52 (94.5%) | 42 (70%) | 0.0006 |
Cerebrospinal Fluid Characteristics
Cell Count, Protein and Glucose Levels
In TBM group, mean CSF cell count was 303.21 cells /mm3, mean CSF protein and glucose were 170.2 mg/dl and 38.3 mg/dl respectively. Statistically significant results were obtained between cases and controls when CSF features of protein >100mg%, cells>20/mm3 & CSF sugar <60% of corresponding blood sugar were compared [Table/Fig-1].
ADA Levels
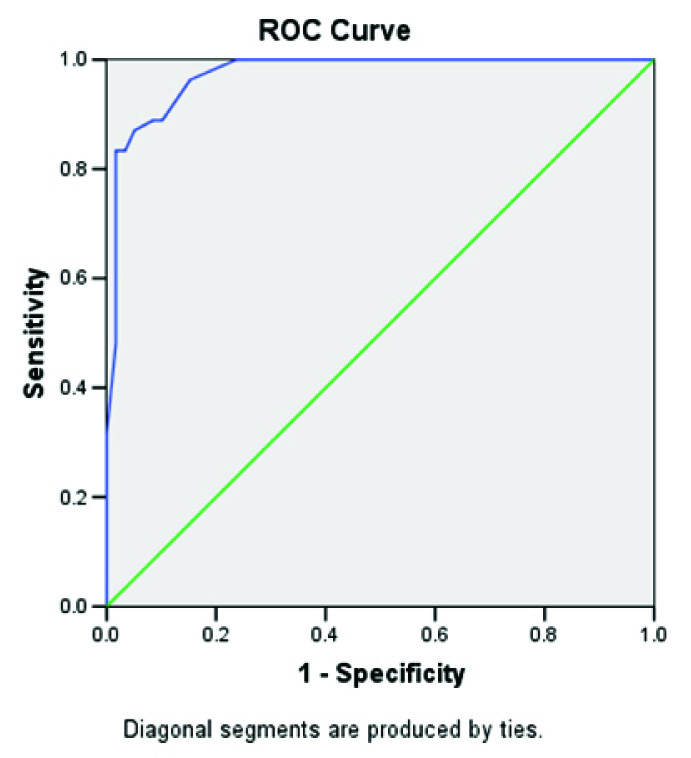
Mean CSF ADA value in TBM patient was 14.44 ± 6.91 IU/L, which was significantly higher than the control group having value of 3.156 ± 2.253 IU/L (p < 0.001). Within the confirmed group, mean value was 20.11± 10.25 IU/L while highly probable, probable and possible TBM groups had values of 13.286 ± 5.45, 13.905 ± 5.68 and 9.333 ± 5.13 IU/L respectively. Mean CSF ADA value in infectious meningitis other than TBM was 3.125 ± 1.907 IU/L and its subgroup pyogenic meningitis had value of 3.142 ± 1.69 IU/L. The TBM patients had significantly higher CSF ADA levels as compared to the control group (p=0.014). A cut off value of 9.5 IU/L showed 83.3% sensitivity and 99.98% specificity by receiver operating characteristic (ROC) curve analysis [Table/Fig-2]. Using this cut off, 81.81% TBM cases showed significant results (p<0.001). All the patients in confirmed and possible TBM group had ADA values > 9.5IU/L while the highly probable group and the probable group had 75% and 79.5% patients with ADA >9.5IU/L.
ROC curve for cut off of ADA
Microbiological Diagnosis
Out of the 55 CSF samples from TBM patients, 2 were positive in Ziehl Neelsen smear (5.55%) and 12 (21.8%) were positive for Mycobacterium tuberculosis in culture (LJ and BACTEC MGIT 960). Multiplex PCR was positive in 45 patients (including HIV positive cases) out of total 55 TBM cases, thereby showing a sensitivity of 81.81%. None of these tests were positive in the control group, thus showing specificity of 100%.
Neuroimaging Findings
CT/MRI findings were suggestive of TBM in 34 patients (61.8%) and the common findings included hydrocephalus (24%), presence of basal exudates (22%), meningeal enhancement (20%), presence of tuberculomas (7%) and presence of infarcts (3.6%). 88.88% of confirmed TBM cases (including HIV positive cases) had the suggestive findings. The presence of hydrocephalus, basal exudates and meningeal enhancement was significantly higher in TBM group (p <0.05).
Staging of TBM and Outcome
50.9% of the patients belonged to the stage II while 36.4% (including 4 HIV positive cases) were in stage III followed by 12.7% patients who were in stage I. The confirmed cases mostly presented in stage III while most of the suspected patients were in stage II [Table/Fig-3]. Mortality was highest in confirmed cases (66.67%) while in suspected cases it was 39.1%. There was increase in mortality as the stage of disease increased; 28.57%, 39.29% and 55% in stage I, II and III respectively. Total mortality seen in TBM cases was 43.63% which is high because most of the patients presented in later stages of TBM.
Category wise staging of patients and their outcome
| Category | Stage (total number) | Responded | Died (Mortality) |
|---|
| Confirmed (n=9) | Stage I (2) | 1 | 1 |
| Stage II (3) | 2 | 1 |
| Stage III (4) | 0 | 4 |
| Total | 3(33.33%) | 6 (66.67%) |
| Suspected (n=46) | Stage I (5) | 4 | 1 |
| Stage II (25) | 15 | 10 |
| Stage III (16) | 9 | 7 |
| Total | 28 (60.9%) | 18 (39.1%) |
| Highly probable (n=4) | Stage I (0) | 0 | 0 |
| Stage II (3) | 2 | 1 |
| Stage III (1) | 0 | 1 |
| Total | 2 (50%) | 2(50%) |
| Probable (n=39) | Stage I (5) | 4 | 1 |
| Stage II (20) | 13 | 7 |
| Stage III (14) | 8 | 6 |
| Total | 25(64.10%) | 14(35.89%) |
| Possible (n=3) | Stage I (0) | 0 | 0 |
| Stage II (2) | 0 | 2 |
| Stage III (1) | 1 | 0 |
| Total | 1 (33.33%) | 2(66.67%) |
| Grand total | | 28+3=31 | 18+6=24 |
Predictors of Mortality
A total of 24 patients (six confirmed and 18 suspected cases) died due to the disease which included all HIV positive cases. The mean age of these patients was 42.45 years and the male to female ratio was 3:1 [Table/Fig-4]. Univariate analysis showed age ≥ 40 year, loss of appetite, loss of weight, evidence of extraneural TB, past history of TB and presence of basal exudates and hydrocephalus in neuroimaging as predictors of mortality in TBM patients [Table/Fig-5]. The factors which were not associated with mortality included clinical features of fever, vomiting, headache, neck stiffness, seizure, altered sensorium, behavioural disturbances, cranial nerve palsies and stage of disease presentation; neuroimaging features of tuberculomas and meningeal enhancement; CSF biochemical analysis findings and microbiological investigation of multiplex PCR. Multinomial logistic regression of the factors revealed age ≥ 40 year (p=0.03), past history of TB (p=0.008), presence of basal exudates (p=0.009) and hydrocephalus (p=0.025) in neuroimaging as significant risk factors for mortality in TBM patients.
Clinical profile of 24 TBM patients who expired
| Clinical profile | Total 24 deaths | % of patients having respective features |
|---|
| Fever | 22 | 91.6% |
| Headache | 16 | 66.66% |
| Vomiting | 12 | 50% |
| Seizures | 8 | 33.33% |
| Anorexia | 17 | 70.8% |
| Altered sensorium | 18 | 75% |
| Neck rigidity | 17 | 70.8% |
| Cranial nerve Palsy | 3 | 12.5% |
| Hemiparesis/ Hemiplegia | 2 | 8.33% |
| Evidence of extra neural tuberculosis | 11 | 45.8% |
| CSF profile |
| CSF sugar <2/3 CBS | 24 | 100% |
| Protein >100 mg% | 21 | 87.5% |
| CSF TLC >20 cells/mm3 | 21 | 87.5% |
| ADA>9.5IU/l | 19 | 79.2% |
| Neuroimaging | 19 | 79.2% |
| Hydrocephalus (communicating or non communicating ) | 9 | 37.5% |
| Basal exudates | 9 | 37.5% |
| Meningeal enhancement | 5 | 20.8% |
| Tuberculomas | 5 | 20.8% |
| Infarcts | 1 | 4.2% |
Predictors of mortality in TBM patients
| Mortality in | Number | Mortality in | Number | p- value | OR (95% CI) | Risk ratio |
|---|
| Age ≥40 years | 15/21 | Age<40 years | 9/34 | 0.001 | 6.94 (2.05-23.41) | 2.69 |
| LOA | 17/31 | No LOA | 7/24 | 0.05 | 2.949 (0.95-9.12) | 1.88 |
| LOW | 18/32 | No LOW | 6/23 | 0.02 | 3.64 (1.13-11.66) | 2.15 |
| EX TB | 11/16 | No EX TB | 13/39 | 0.01 | 4.4 (1.26-15.34) | 2.06 |
| H/O TB | 8/9 | No H/O TB | 16/46 | 0.003 | 15 (1.72-130.8) | 2.55 |
| Basal exudates | 9/12 | No basal exudates | 15/43 | 0.01 | 5.6 (1.31-23.85) | 2.15 |
| Hydrocephalus | 9/13 | No hydrocephalus | 15/42 | 0.03 | 4.05 (1.06-15.4) | 1.93 |
LOA-loss of appetite, LOW- loss of weight, EX TB- evidence of extraneural TB, H/O TB- History of TB
Discussion
TBM needs to be diagnosed and treated early as it is associated with high mortality and severe neurological sequelae especially in endemic countries like India. Nonspecific symptoms of TBM pose a challenge for the accurate timely diagnosis [8]. The study was carried out to find out the demographics of the disease and factors associated with outcome of the disease in adult TBM patients in northern region of India. 7.27% of the cases were co-infected with HIV in the present study. The data regarding the prevalence of TBM in HIV is scarce and scattered. In a study by Karande et al., in children <12 y of age in India, 6.5% TBM patients were reported have HIV coinfection [9].
The age distribution of the test and control groups was well matched in the present study. The age distribution is similar to study by Thwaites et al., where median age was 34 y in cases and 41 y in controls [10]. In another study, median age of cases was 22 y and that of controls was 24.5 y [11]. The disease was found to be more common in males. No significant difference in sex ratio was observed between cases and controls in the present study. The clinical picture of TBM is hard to differentiate from other types of meningitis. There are certain features like long history of illness (>5-6 d) which predict TBM [8,10,12]. In our study, all the cases had history of at least 14 d including fever (90.9%), headache (72.7%) and vomiting (54.5%). The triad of these symptoms was present in 60% of the patients. The occurrence of these symptoms was similar or higher as compared to earlier studies [1,8,13, 14]. This may be due to strict inclusion criteria of fever and headache in study group. The incidence of seizures was slightly higher in this study and that of hemiparesis, cranial nerve abnormalities and presence of extraneural TB manifestations were lower as compared with the earlier studies [8,10]. The most common cranial nerve involved was the 6th nerve (9%) which is consistent with the findings of earlier studies where 6th nerve has been reported to be most commonly involved in TBM [15]. Neck rigidity seen in different studies ranged from 18% to 91% [8,10]. In the present study, it was found out to be 67.3% thus comparable with the earlier studies. The presence of neck rigidity, extraneural TB and past history of TB were statistically significant when compared with the control group. Patients were clinically divided into 3 stages using Medical Research Council staging system [7]. Maximum patients were in stage II (n=28) followed by stage III (n=20) and stage I (n=7). Similar staging has been done in earlier studies also [16]. Higher number of stage II and stage III TBM patients in our study may be explained by the fact that ours is a tertiary care institute and patients are usually referred here for management from peripheral hospitals. The HIV positive cases had similar symptoms as HIV negative patients although they presented in later stage of disease and had higher frequency of extraneural presentation as reported earlier [17]. In a retrospective study conducted in Turkey, 160 cases of TBM were evaluated for epidemiological, clinical, laboratory and neuroimaging features [18]. The commonest clinical features were headache, fever, vomiting and neck stiffness as seen in our study while 63% patients presented in stage II and 21% in stage III. However, culture positivity was high in the study (39.9%).
The CSF biochemical picture is consistent with the usual observation of CSF findings suggestive of TBM seen in various studies also [8,19]. In the study carried out by Christensen et al., 86% had elevated protein values, 90% had elevated WBC count and 50% patients had CSF: blood glucose ratio of <0.33 [8]. In many review articles, the CSF picture has been said to be having pleocytosis of more than 20 cells/mm3, proteins >100mg/dl and CSF sugar less than 60% of corresponding blood sugar [15]. The difference in the mean CSF ADA values between TBM patients and Non TBM infectious meningitis was also found to be statistically significant. (p=0.014). The cut off value of 9.5 IU/L was crossed by 81.81% of the cases as compared to the controls where only one patient (1/60, 1.66%) of Japanese encephalitis had this higher value. This finding was significant with a p-value of <0.001. Similar finding has been reported in earlier studies also [20,21]. Earlier, various studies reported the reliability of CSF ADA activity in TBM patients using different cut off values [19]. Our results are in accordance with study of Gambhir et al., who found mean CSF-ADA activities in TBM patients significantly higher (9.6 ± 4.1 IU/L) than those suffering from viral meningitis or non-infectious etiology [22]. Moghtaderi et al., found mean CSF-ADA activity in TBM group (23.05±13.1 IU/L) to be significantly higher than in the CSF from non-TBM ( pyogenic plus viral ) infectious meningitis patients (9.39±5.18 IU/L) [23]. The highest accuracy was observed when cut-off value of 10.5 IU/L was considered. The sensitivity and specificity of the test to differentiate TBM from non-TBM was found to be 81% and 86% respectively. Karsen et al., found with cut-off value of 12.35 IU/L for differential diagnosis of TBM and pyogenic meningitis, sensitivity was 92% and specificity was 100% [24]. However, cut-off level of 11 IU/L for differentiating between TB and non-TB by applying ROC analysis was found to have sensitivity of 92% and specificity of 90%. Gupta et al., indicated that ADA level in CSF with 10 IU/L as a cutoff value demonstrated 94.73% sensitivity and 90.47% specificity in differentiating TB from non-TBM [20]. Sun et al., concluded that ADA estimation with a cut off value of 9.5 IU/L in CSF was an adjunct for the differential diagnosis of TBM and non-TBM [21]. Our finding was consistent with this recent observation for using 9.5 IU/L as a cut off value.
On neuroimaging, hydrocephalus (n=13) (24%) was the most common finding observed, followed by basal exudates (n=12) (22%), meningeal enhancement (n=11) (20%) tuberculomas (n=4) (7%) and infarcts (n=3) (3.6%). Christensen et al., in his study on Danish people also found hydrocephalus as the most common finding (29%) [8].They also had high incidence of infarcts (29%) and tuberculomas (14%). While in the immigrant population, basal meningeal enhancement (67%) was the most common finding. In various reviews, hydrocephalus and basal meningeal enhancement have been reported to be the most common finding on neuroimaging [15]. However, the most common imaging feature was tuberculomas and basal meningitis in a study conducted in Turkey [18]. Higher frequency of hydrocephalus in our institute may be due to the referral bias of tertiary care centre, as mentioned earlier.
In the present study, it was observed that with increasing stage i.e. from I to III, the number of patients who responded to treatment gradually decreased and mortality increased. Most of the cases in confirmed category belonged to stage III (44.4%) while most of the cases in suspected group belonged to stage II (54.3%). Total mortality seen in TBM cases was 43.63% which is high because most of the patients presented in later stages of TBM. All HIV positive cases died showing high rates of mortality in HIV-TBM coinfection. The mortality rate in a study in Denmark has been reported to be 19% [8]. Studies in areas of endemic TB have shown higher mortality rate (69% in South Africa and 67% in Vietnam) [25,26]. Severity of TBM at presentation serves as a good prognostic marker. This observation is in league with the previous studies which had identified the most important determinant of outcome for both survival and sequelae being the neurological stage of the disease at which treatment was started. If the treatment was started during early stages, the mortality and morbidity was low. However, in stage III the mortality was almost 50% and most of the survivors suffered from some form of neurological deficit [27,28].
Age ≥ 40 y, loss of appetite, loss of weight, evidence of extraneural TB, past history of TB and presence of basal exudates and hydrocephalus were observed as predictors of mortality in our study. In a study by George et al., age > 40 y, Glasgow coma scale (GCS) score <8, CSF protein ≤60 mg% and MRC stage III were found to be the predictors of mortality in TBM [6]. Similar to above results, a study conducted in Taiwan also showed age >60 years as an important factor associated with higher mortality [29]. Girgis et al., have shown presence of hydrocephalus to be a risk factor for poor outcome of TBM [30]. Similar to this finding we also observed higher rate of mortality in patients with hydrocephalus. Many studies have shown absence of headache as a predictor of mortality which may be due to the delay in presentation in such cases [6,29]. Such observation was not there in the present study. Delayed treatment, female gender, infarction, low CSF glucose levels, low CSF/blood glucose ratio and high CSF protein concentration are the factors which have been observed to predict mortality in TBM cases in various studies but univariate analysis in our study did not reveal such association with these variables [4,26,29,31,32].
Conclusion
Thus, the present study provides the epidemiological factors of TBM in adult patients in Northern India including clinical, laboratory and imaging data. Our results are comparable to various studies in developing countries. The presence of TB at sites other than CNS, associated pulmonary TB and positive imaging findings have highlighted the importance of radiodiagnosis in our study. CSF ADA value of >9.5IU/L and multiplex PCR have shown a good sensitivity and specificity for diagnosis of TBM. It is a preliminary study with small number of study subjects. Multicentric studies regarding the issue in future may help in better exploration of the disease and associated factors. The present study may help in formulation of mortality prediction score using age>40 y, imaging findings of hydrocephalus and basal exudates and past history of TB as variables. This will improve the early implementation of stringent management therapies to save the patient.