Objective: To evaluate the effect of Edrophonium on blood glucose levels in euglycemic albino rats through OGTT.
Materials and Methods: Twelve Swiss albino rats weighing around 150-200 gms of either sex were randomly selected from the central animal facility, JSSMC, Mysore and divided into two groups. The control group received distilled water (25ml/kg body wt.) per orally, test groups received Edrophonium (6.3mg/kg/day) intravenously for five days. On the fifth day, following overnight fasting, half an hour after drug administration in all the groups of rats Oral Glucose Tolerance Test was performed, by administering oral glucose in dose of 0.6gm/kg body weight. The capillary blood glucose levels were measured at 0, 60 and 150 min, by rat tail snipping method using (ACCUCHEK) glucometer.
Results: The Capillary Blood Glucose levels of Edrophonium group was less when compared to control group at all-time intervals.
Conclusion: Edrophonium showed the hypoglycemic activity when given for five days intravenously in euglycemic albino rats through Oral Glucose Tolerance Test.
Introduction
Diabetes mellitus (DM) consists of a group of syndromes characterized by hyperglycemia, altered metabolism of lipids, carbohydrates and proteins and an increased risk of complications from vascular and neurological abnormalities. Most patients are classified as having either type 1 or type 2 diabetes mellitus.
Increased hepatic glucose output predominately accounts for increased fasting levels, whereas decreased peripheral glucose utilization results in post prandial hyperglycemia. Type 2 diabetes is characterized by two fundamental defects: insufficient production of insulin by pancreatic β-cells and reduced target-tissue sensitivity to the effects of insulin (insulin resistance) [1].
Type II DM is one of the most challenging health care problems, which requires optimum management. At present the treatment of type 2 diabetes mellitus includes insulin, sulfonylureas, biguanides, α-glucosidase inhibitors, DPP-4 inhibitors, thiazolidinedione’s, GLP-1 receptor agonists, amylin agonists, medical nutrition therapy and lifestyle modification [2].
Insulin Release:- Entry of glucose is phosphorylated by glucokinase to glucose 6 phosphate, which enters the glycolytic pathway, producing NADPH and the ratio of ATP/ADP is increased. Elevated ATP/ADP inhibits ATP sensitive K+ channel leading to depolarization. The ATP sensitive K+ channel consists of (kir6.2) inward rectifying channel and a closely related (SUR) sulfonylurea receptor. The closure of K-ATP channel depolarizes the cell membrane to potential above -55 mV. This leads to the activation of T-type (at voltages above -60 mV), L-type Ca2+ channels (above -50 mV) and initiates the action potential. During the upstroke of the action potential, voltage-gated Na+ channels open (above -40 mV), leading to further acceleration of the upstroke and sufficient depolarization activate P/Q-type Ca2+ channels (above -20 mV). Ca2+ influx via P/Q-type (and to a lesser extent L-type) directly triggers exocytosis of insulin granules. Na+ current is important for action potential generation and glucose-induced insulin secretion.
Voltage-gated plasmalemmal ion channels play a fundamental role in stimulus secretion coupling in cells, and Ca2+ influx through voltage-gated Ca2+ channels triggers exocytosis of insulin containing secretory granules. Voltage-gated Ca2+ channels are activated by coordinated fluctuations of the cell membrane potential (electrical activity), which are initiated by the glucose-induced closure of ATP-sensitive K+ channels (KATP channels) and dependent on voltage- gated Na+ and K+ channels [3].
Oral Glucose Tolerance Test (OGTT): -The OGTT is a measure of the glucose induced insulin secretion and its mediated glycemic changes. This study used OGTT for normoglycemic rats with some modifications to the standard method to assess the effect of Edrophonium on glucose induced glycemic alteration [4].
Edrophonium is a shortest acting reversible acetyl cholinesterase inhibitor. It prevents breakdown of the neurotransmitter acetylcholine and acts by competitively inhibiting the enzyme acetyl cholinesterase mainly at the neuromuscular junction. Currently, Edrophonium is used for diagnosis of myasthenia gravis, reversal of non depolarizing neuromuscular block and in treatment of respiratory depression caused by over dosage of non-depolarizing neuromuscular blocking agents. Edrophonium has a short half-life of 7-12 min and eliminated through kidney [5].
The effect of acetylcholine/ vaguson pancreatic insulin release is mediated through activation of muscarinic acetylcholine receptors located on the pancreatic β-cells.
Pancreatic polypeptide (PP) is produced by the F-cells of the pancreas and is secreted by cholinergic stimulation. In the gastrointestinal tract, PP inhibits gastric emptying rate and gallbladder motility, decreases food intake and increases energy expenditure. Alterations in circulating PP levels are reported in several states of glucose intolerance, such as Type 1 Diabetes Mellitus and type 2 Diabetes Mellitus. PP enhances insulin sensitivity and reduces insulin requirements in patients with long-standing Type 1DM and Type 3cDM. Type 3c DM category includes diabetes in association with, or as a consequence of, acute and chronic pancreatitis (CP), pancreatic neoplasms, pancreatic resection, pancreatic trauma, fibro calculous pancreatopathy, cystic fibrosis, hemochromatosis, and pancreatic agenesis is caused due to chronic pancreatitis and pancreatic resection. PP is an important modulator of peripheral insulin action [6,7].
Edrophonium has excitatory effect on neural tissue leading to increased neural activity. Through its cholinergic stimulation it causes increase in PP.
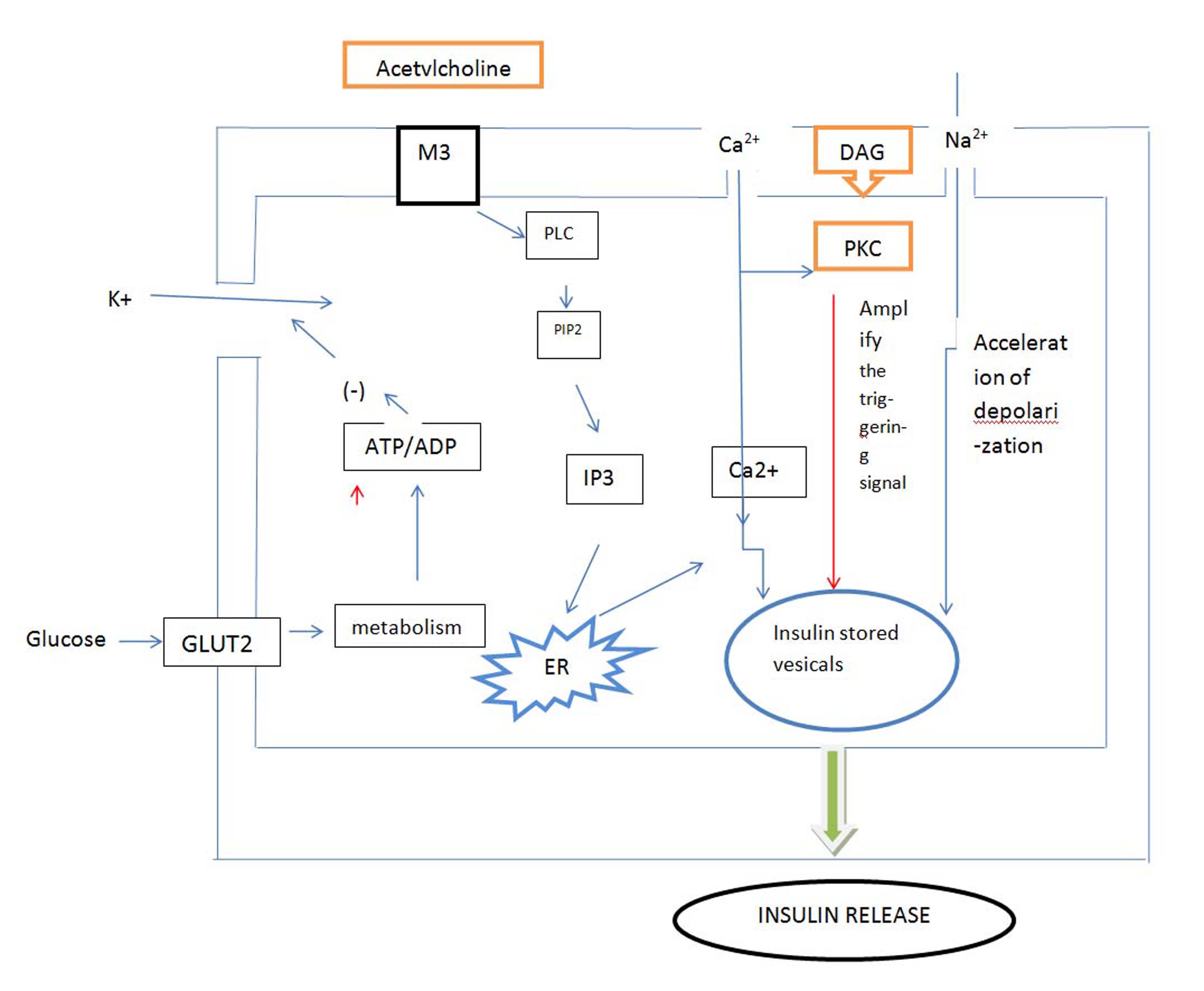
Cholinergic M3 receptors are present in visceral smooth muscles, iris, ciliary muscle, exocrine glands, endocrine glands and vascular endothelium [8]. Edrophonium produce actions similar to that of acetylcholine by increasing availability of acetylcholine at these sites (anticholinesterase) and make acetylcholine to act on cholinergic receptors [9]. The sequential events are depicted in the [Table/Fig-1].
Shows the schematic representation of mechanism of action of insulin release through muscarinic receptors (M3) on pancreatic β cell
M3:-Muscarinic M3 Receptors; PLC:-Phospholipase C; PIP2:-Phosphatidylinositol 4, 5- bisphosphate; IP3:-Inositol triphosphate; ER:-Endoplasmic reticulum; DAG: -Diacyl glycerol; PKC: - Phosphokinase C.
Hence, Edrophonium may work as a meal medication in DM. It control meal mediated hyperglycemia as add on therapy, if not effectively been controlled by post prandial control protocol treatment.
Thus the mechanism by which cholinomimetics acts to cause insulin secretion is,
Major pathways are activation of PLC, which mainly generates IP3 and diacylglycerol, a potent PKC activator [10].
Acetylcholine also depolarizes the plasma membrane of βcells by a Na+ or nonspecific cationic-dependent mechanism.
HYPOTHESIS: Acetylcholine acting through M3 receptors, by activation of phospholipase C generates IP3 and diacylglycerol. It depolarizes the membrane of insulin stored granules by sodium channel and causes secretion of insulin, therefore decrease in blood glucose. Also Edrophonium enhances insulin sensitivity and reduces insulin requirements in patients with long-standing Diabetes Mellitus by increasing pancreatic polypeptide.
Materials and Methods
The study was conducted after the approval of IAEC (Institution Animal Ethical Committee). CPSEA approval number from IAEC of: JSSMC/IAEC/12/5655/DEC 2013
Albino rats of either sex of average weight 150-200gms aged 3-4 months were used in experiments. The albino rats were bred in Central Animal Facility of JSS Medical College, Mysore. The study was done in Department of Pharmacology during the month of March 2014. Animals were acclimatized to the laboratory conditions for at least one hour before testing and then they were used during experiments. The doses of drugs were based on the human daily dose converted to that of animal dose using the standard formula [11].
Drugs and chemicals:- Inj. Edrophonium 6.3mg/kg/day (Baxters, India) was administered intravenously, distilled water given orally, 0.6mg/kg of glucose mixed in distilled water for OGTT.
The rats were divided into 2 groups with 6 animals (n=6) in each group (control and test group). The test drug Edrophonium 6.3mg/kg/day was administered intravenously and distilled water 25ml/kg/day was administered orally for five days.
Group 1:- Distilled water- 25ml/kg/day (orally)
Group 2:- Edrophonium-6.3mg/kg/day (I.V)
All rats were fasted overnight before the 5th day. On the 5th day half an hour after the last dose of the respective drug, OGTT was performed. All the rats were given glucose (0.6gm/kg body weight) orally using gavage tube. The Capillary blood glucose levels (obtained by tail snipping) were assessed at 0, 60, and 150 min of time intervals using a glucometer (ACCUCHEK).
Statistical Analysis
The results were analysed by calculating the Mean values, Standard Deviation, t- Test and the analysis of variance (ANOVA) at different time intervals within the same group, followed by independent sample t-test between the two groups. The values were compared at 0.05 level of significance to test the results of the study for the corresponding degrees of freedom. p<0.05 was considered as significant. All the statistical analysis was done by using IBM SPSS 21 software.
Results
Edrophonium group showed fall in blood glucose level throughout the OGTT compared to control with the maximum fall at 60 min.
Discussion
In normal individuals, the insulin release from β cells through the early phase begins within minutes of a glycemic stimulus. Early-phase insulin primes tissues that are sensitive to site, in particular liver, which results in the reduction of hepatic glucose output. In type 2 diabetic patients the important defect in insulin secretion is the impairment of early-phase insulin release which is both delayed and blunted [12].
In Impaired Glucose Tolerance (IGT), basal insulin secretion is normal but glucose-stimulated first- and second-phase insulin secretion and (peripheral) insulin sensitivity is reduced. The loss of early-phase insulin release during and after the prandial phase has several deleterious effects on normal glucose homeostasis: hepatic glycogenolysis and gluconeogenesis are not inhibited sufficiently, and glucose uptake by muscle is insufficient. This leads to the postprandial hyperglycemia observed in glucose-intolerant and type 2 diabetic patients.
In pancreatic βells, glucose stimulates insulin secretion by generating, triggering and amplifying signals. The triggering pathway is well characterized. It involves the following sequence of events: entry of glucose by facilitated diffusion, metabolism of glucose by oxidative glycolysis, rise in the ATP-to-ADP ratio, closure of ATP-sensitive K+ (KATP) channels, membrane depolarization, opening of voltage-operated Ca2+ channels, Ca2+ influx, rise in cytoplasmic free Ca2+ concentration ([Ca2+]i), and activation of the exocytotic machinery. Under these conditions, glucose still increases insulin secretion in a concentration-dependent manner. This increase in secretion is highly sensitive to glucose (produced by as little as 1–6 mmol/l glucose), requires glucose metabolism, is independent of activation of protein kinases A and C, and does not seem to implicate long-chain acyl-CoAs. Changes in adenine nucleotides may be involved. The amplification consists of an increase in efficacy of Ca2+ on exocytosis of insulin granules. There exists a clear hierarchy between both pathways. The triggering pathway predominates over the amplifying pathway, which remains functionally silent as long as (Ca2+]i has not been raised by the first pathway; i.e., as long as glucose has not reached its threshold concentration. The amplifying pathway serves to optimize the secretory response not only to glucose but also to non-glucose (amino acids) stimuli. It is impaired in β-cells of Type 2 diabetic patients. Besides the available drugs that act on K+ATP channels and increase the triggering signal, novel drugs that correct a deficient amplifying pathway would be useful to restore adequate insulin secretion in type 2 diabetic patients [13,14].
OGTT is an established method to prove the intactness of β cells and influence of glucose on glucose stimulated insulin secretion which is depicted at periphery by variation of glycemic levels in the blood.
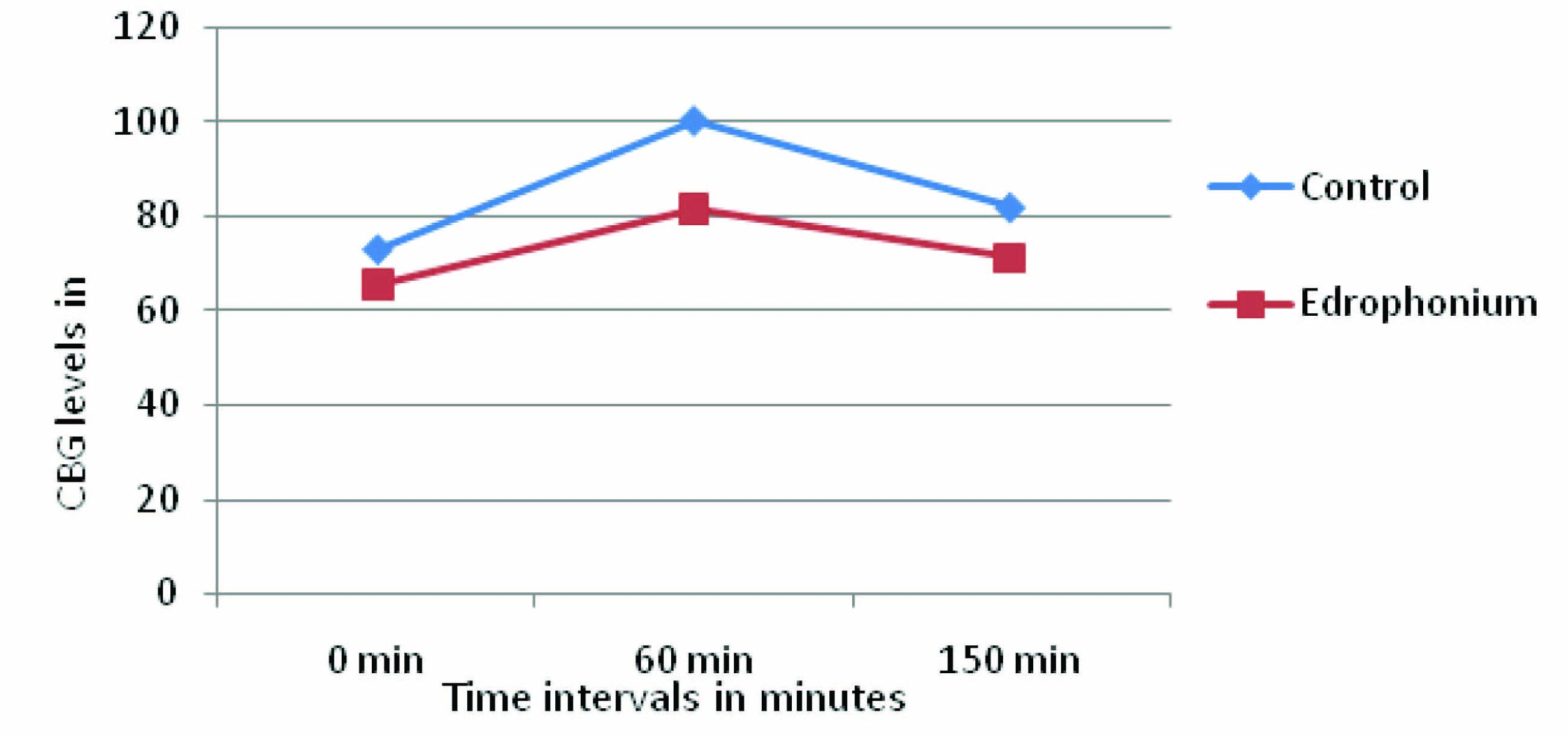
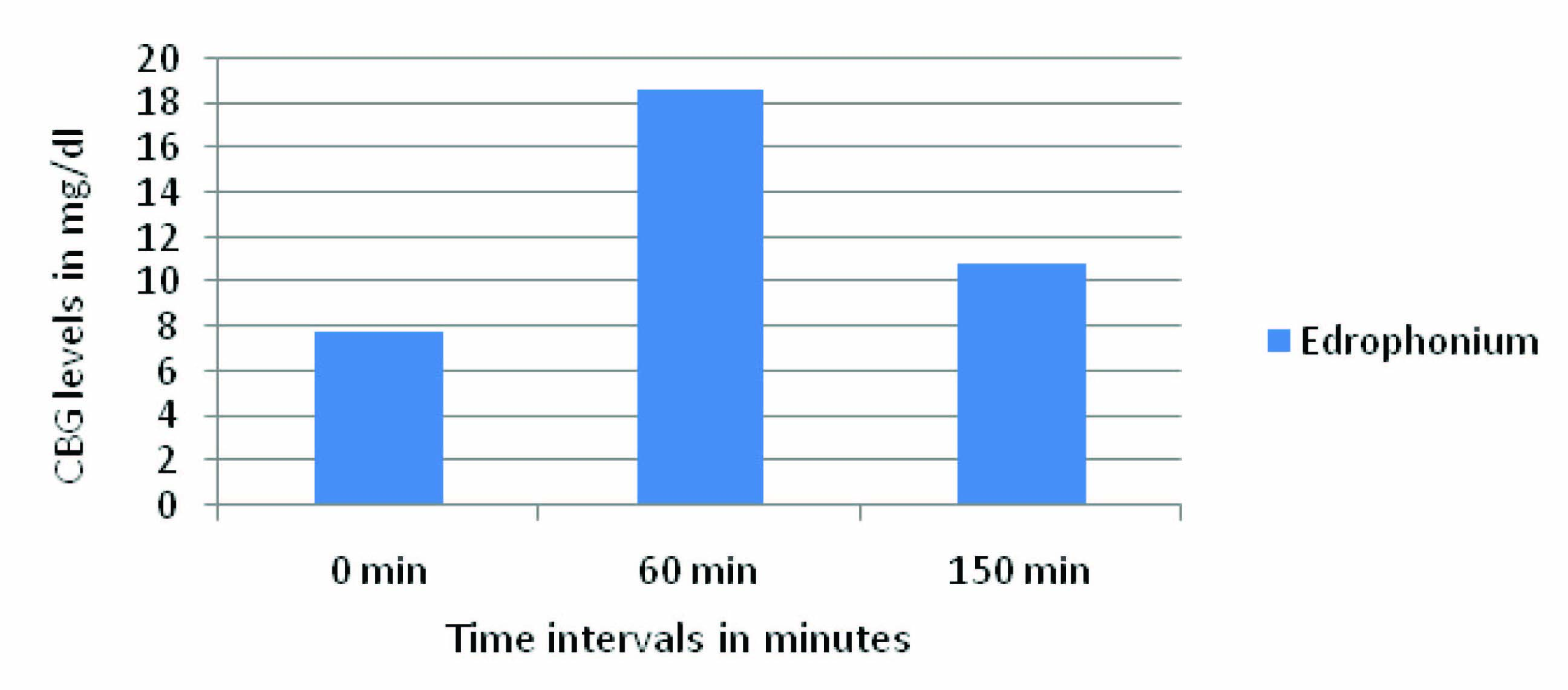
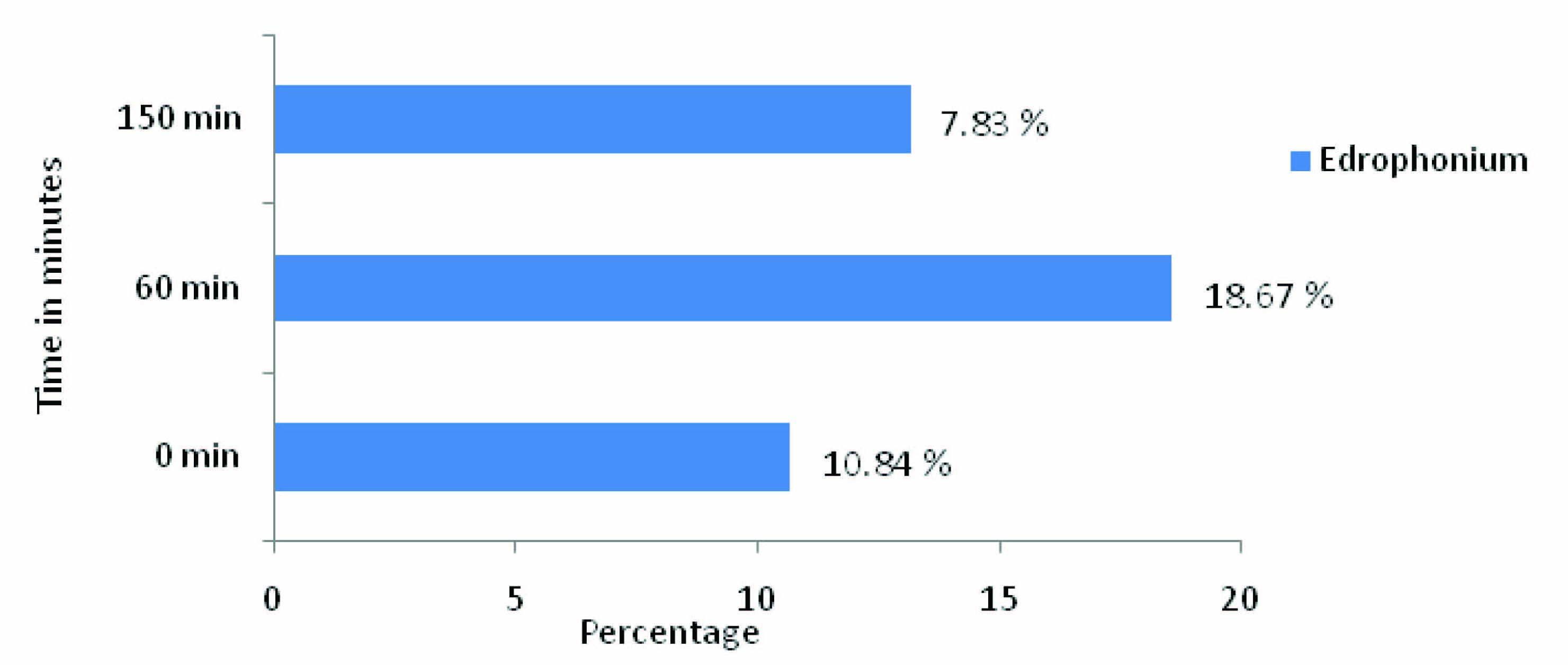
In the present study, in [Table/Fig-2,3] Edrophonium group showed decrease in the Capillary blood glucose (CBG) levels at all-time intervals of OGTT i.e.,0 min, 60 min, and 150 min when compared to control. The CBG level at 0 min is 7.83±1.8 more when compared to control i.e., 10.7%, which indicates indirectly, that Edrophonium increased basal secretion of insulin. The CBG level at 60 min is 18.67±1.08 more when compared to control i.e.,18.6% which indicates that Edrophonium causes more glucose dependent insulin secretion from pancreatic cells.The CBG level at 150 min is 10.84±0.68 more when compared to control i.e., 13.21 % because of sustained action of Edrophonium on pancreatic β cells. The quantum of fall in CBG levels of Edrophonium group at 0 min and 150 min is almost equal. [Table/Fig-4] showed the Capillary Blood glucose (CBG) levels and difference between control, and Edrophonium group. [Table/Fig-5,6] showed the Reduction in CBG levels and Percentage fall in Capillary Blood glucose (CBG) of Edrophonium group compared to control group.
Capillary Blood glucose (CBG) levels in control, and Edrophonium group and the difference between the control and bethanecol group at corresponding time intervals.
| Time interval during OGTT | Capillary Blood glucose concentration in mg/dl |
|---|
| Control group n=6 | Edrophonium group n=6 | Fall in CBG levels Edrophonium group compared to control group |
|---|
| 0 min | 73.16 ±4.87 | 65.33±3.07 | 7.83±1.8 |
| 60 min | 100.33±3.55 | 81.66±4.63* | 18.67±1.08 |
| 150 min | 82±4.19 | 71.16±4.87* | 10.84±0.68 |
Data is expressed as mean ± SD of n=6, *p<0.05 compared with control (distilled water). SD: Standard Deviation
Percentage fall in Capillary Blood glucose (CBG) level in Edrophonium group when compared to control
| S. no | 0 min | 60 min | 150 min |
|---|
| Edrophonium | 10.70 | 18.60 | 13.21 |
Capillary Blood glucose (CBG) levels in control, and Edrophonium group and the difference between the control and Edrophonium group
Reduction in CBG levels of Edrophonium group compared to control group
Percentage fall in Capillary Blood glucose (CBG) concentration in Edrophonium group when compared to control
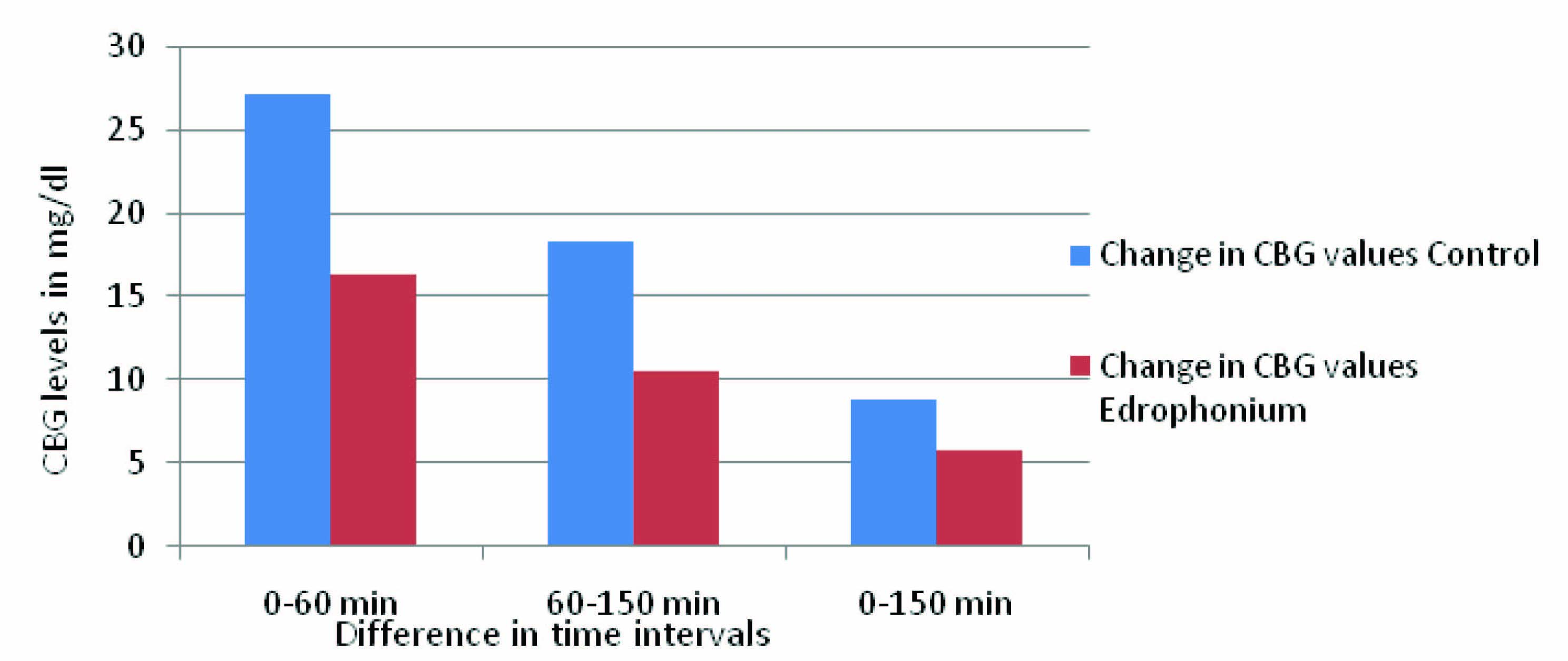
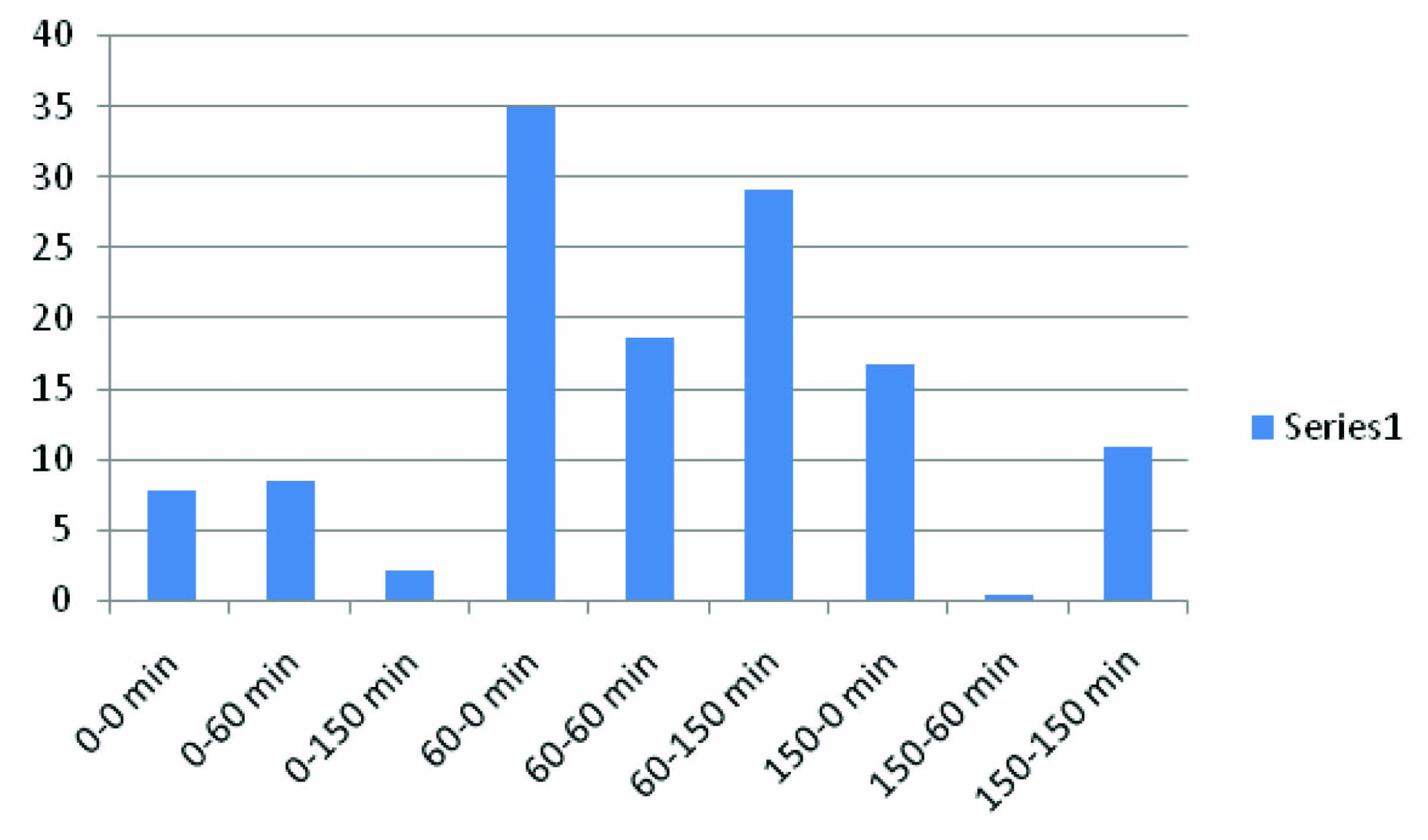
In [Table/Fig-7,8], the interval difference of bethanecol group at 0-60 min is maximum, which indicates glucose dependent insulin release and inter-interval difference at 60-150 min of bethanecol group is more compared to 0-150 min because of sustained effect on pancreatic β cell. The interval difference at 0-150 min is the total combined effect of bethanecol on pancreatic β cells. [Table/Fig-9] showed the difference in the CBG levels of Edrophonium and control group at time intervals 0 min, 60 min and 150 min of OGTT. [Table/Fig-10] showed difference in CBG values between control group and Edrophonium group at various time intervals.
The difference in the CBG levels of Edrophonium and control group at time intervals 0 min, 60 min and 150 min of OGTT
| Sl no | Time interval | Change in CBG values |
|---|
| Control | Edrophonium |
|---|
| 1 | 0-60 min | 27.17±1.32 | 16.33±1.32* |
| 2 | 60-150 min | 18.33±0.64 | 10.5±0.24* |
| 3 | 0-150 min | 8.84±0.68 | 5.83±1.8* |
Data is expressed as mg/dl, *p<0.05 compared with control (distilled water)
Depicts difference in CBG values between control group and Edrophonium group at various time intervals
| S. no | OGTT time interval of Control - OGTT time interval of Edrophonium (C- T) | Difference in CBG values (mg/dl) |
|---|
| 1 | 0-0 min | 7.83±1.8 |
| 2 | 0-60 min | 8.5±0.24* |
| 3 | 0-150 min | 2 |
| 4 | 60-0 min | 35±0.48* |
| 5 | 60-60 min | 18.67±1.08* |
| 6 | 60- 150 min | 29.17±1.32 |
| 7 | 150-0 min | 16.67±1.12* |
| 8 | 150-60 min | 0.34±0.44 |
| 9 | 150-150 min | 10.84±0.68* |
Data is expressed as mg/dl, *p<0.05 compared with control (distilled water)
The difference in the CBG levels of Edrophonium and control group at time intervals 0 min, 60 min and 150 min of OGTT
Difference in CBG values between control group and Edrophonium group at various time intervals
The above finding indicates that Edrophonium acts as a hypoglycemic drug in normal albino rats. OGTT is used to assess the glucose tolerance which indirectly indicates the insulin sensitivity and beta cell function [15]. Hence, in diabetics and pre-diabetics it can be assumed that Edrophonium causes decreased in blood glucose levels.
Conclusion
The test drug Edrophonium showed significant decrease in capillary blood glucose level in euglycemic albino rats when compared to that of control through OGTT. The hypoglycemic activity of Edrophonium is maximum at the 60th min, which justifies the hypothesis stated above and enhanced the glucose dependent insulin release.
Thus, to conclude Edrophonium causes decrease in blood glucose levels in euglycemic albino rats through muscarinic receptor stimulation, through activation of phospholipase C generates IP3, diacylglycerol also, depolarizes membrane by sodium channel and causes secretion of insulin. And, through its cholinergic stimulation it causes increase in Pancreatic Polypeptide. Therefore, decrease in blood glucose.
Data is expressed as mean ± SD of n=6, *p<0.05 compared with control (distilled water). SD: Standard Deviation
Data is expressed as mg/dl, *p<0.05 compared with control (distilled water)
Data is expressed as mg/dl, *p<0.05 compared with control (distilled water)
[1]. Abbruzzese James L, Aboulhosn Jamil, Achermann John C, Powers Alvin C, James Peter, Diabetes mellitus. In Dan L. Longo, Dennis L. Kasper, editorsHarrison’s princples of internal medicine 2013 New YorkMcgraw- hill:2276-79. [Google Scholar]
[2]. Nicholson G, Hall G.M, Diabetes mellitus: new drugs for a new epidemicBritish Journal of Anaesthesia 2011 107(1):65-73. [Google Scholar]
[3]. Matthias Braun, Ramracheya Reshma, Bengtsson Martin, Voltage-Gated Ion Channels in Human Pancreatic β Cells: Electrophysiological Characterization and Role in Insulin SecretionOxford Centre for Diabetes Endocrinology and Metabolism. Diabetes 2008 57:1622-27. [Google Scholar]
[4]. Breda E, Cavaghan MK, Toffolo G, Polonsky KS, Cobelli C, Oral glucose Tolerance Test minimal Model Indexes of β-cell Function and Insulin SensitivityDiabetes 2001 50:150-58. [Google Scholar]
[5]. Hilal-Dandan R, Muscarinic receptor agonist and antagonist. In: Bruton LL, editorGoodman and Gilman’s the Pharmacological Basis of Therapeutics 2011 12th edChinaMcGraw Hill:311-25. [Google Scholar]
[6]. Juraj Koska, Angelo DelParigi, Barbora de Courten, Christian Weyer, Tataranni P Antonio, Pancreatic Polypeptide Is Involved in the Regulation of Body Weight in Pima Indian Male SubjectsDiabetes 2004 53:3092-96. [Google Scholar]
[7]. Rabiee Atoosa, Galiatsatos Panagis, Salas-Carrillo Rocio, Thompson Michael J, Andersen Dana K, Elahi Dariush, Pancreatic Polypeptide Administration Enhances Insulin Sensitivity and Reduces the Insulin Requirement of Patients on Insulin Pump TherapyJournal of Diabetes Science and Technology 2011 5(6):1521-28. [Google Scholar]
[8]. Gautam D, Han SJ, Duttaroy A, Mears D, Hamdan F, Li JH, Role of the M3 muscarinic acetylcholine receptor in β-cell function and glucose homeostasisDiabetes, Obesity and Metabolism 2007 9(2):158-69. [Google Scholar]
[9]. Daniela Billups, Brian Billups, Challiss RA John, Nahorski Stefan R, Modulation of Gq-Protein-Coupled Inositol Trisphosphate and Ca2 Signaling by the Membrane PotentialThe Journal of Neuroscience 2006 26(39):9983-95. [Google Scholar]
[10]. Henquin Jean Claude, Kahn C Ronald, Weir Gordon C, King George L, Jacobson Alan M, Smith Robert J, Cell Biology of insulin secretion. In: C Ronald Kahn, Gordon C Weir, editorsJoslin’s diabetes mellitus 2006 NoidaLippincott Williams and Wilkins:86-95. [Google Scholar]
[11]. Medhi Bikash, Prakash Ajay, Introduction to experimental pharmacology. In: BikashMedhi, editorsPractical manual of experimental and clinical pharmacology 2010 New DelhiJaypee:23-25. [Google Scholar]
[12]. Tuomilehto J, Point: a glucose tolerance test is important for clinical practiceDiabetes Care 2002 25:1880-82. [Google Scholar]
[13]. Henquin Jean-Claude, Triggering and Amplifying Pathways of Regulation of Insulin Secretion by GlucoseDiabetes 2000 49:1751-60. [Google Scholar]
[14]. Mitsuhisa Komatsu, Yoshihiko Sato, Satoko Yamada, Keishi Yamauchi, Kiyoshi Hashizume, Toru Aizawa, Triggering of Insulin Release by a Combination of cAMP Signal and Nutrients An ATP-Sensitive K Channel–Independent PhenomenonDiabetes 2002 51(1):29-32. [Google Scholar]
[15]. Meier Juris J, Menge Bjoern A, Breuer Thomas GK, Müller Christophe A, Tannapfel Andrea, Uhl Waldemar, Functional Assessment of Pancreatic Cell Area in HumansDiabetes 2009 58:1595-603. [Google Scholar]