Angiogenesis in Breast Cancer and its Correlation with Estrogen, Progesterone Receptors and other Prognostic Factors
Jyotsna Naresh Bharti1, Poonam Rani2, Vinay Kamal3, Prem Narayan Agarwal4
1 Senior Resident, Department of Pathology, Maulana Azad Medical College, New Delhi, India.
2 Senior Resident, Department of Pathology, Maulana Azad Medical College, New Delhi, India.
3 Director Professor, Department of Pathology, Maulana Azad Medical College, New Delhi, India.
4 Director Professor & HOD, Department of Surgery, Maulana Azad Medical College, New Delhi, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Jyotsna Naresh Bharti, Senior Resident, Department of Pathology, Maulana Azad Medical College, Bahadur Shah Zafar Marg, New Delhi 110002, India. E-mail : jyotsnamamc@gmail.com
Purpose: The aim of study is to evaluate angiogenesis using CD34, in estrogen, progesterone positive and negative breastcancer and to correlate the microvessel density with known histological prognostic factors, morphological type of breast carcinoma and lymph node metastasis.
Materials and Methods: Twenty eight untreated cases of breast cancer were included in the study and paraffin embedded sections were obtained from representative mastectomy specimen of breast cancer patient. The sections were stained with hematoxylin and eosin stain and immunohistochemistry was performed using CD34, estrogen, progesterone, cytokeratin and epithelial membrane antigen antibody. Angiogenesis was analysed using CD 34 antibody. For statistical analysis, cases were grouped into estrogen, progesterone positive and negative receptors.
Results: Mean microvessel density in ER-/PR-, ER-/ PR+, ER+/PR-, ER+/PR+ was 15.45, 14.83, 11, 10.89 respectively. A significant correlation was found between ER receptors and mean vascular density with p-value (< 0.05). A significant difference was observed in mean vascular density between the four groups comprising (p-value < 0.05). Infiltrating duct carcinoma (NOS) grade III has got the highest mean microvessel density (14.17) followed by grade II (12.93) and grade I (12.33).
Conclusion: Information about prognostic factors in breast cancer patients may lead to better ways to identify those patients at high risk who might benefit from adjuvant therapies.
CD 34 antibody, ER, Immunohistochemistry, Microvessel density, Mastectomy
Introduction
There has been global increase in incidence of breast cancer over the last several decades in Asian countries than in United States and Europe [1]. Biological and epidemiological profiles of breast cancer vary globally. Breast cancer is more frequent in Asia pacific region is young premenopausal women with peak at 40 to 50 y as compared to west and more frequently diagnosed in advanced stages and likely to be estrogen receptor and progesterone receptor negative [2–4]. A prognostic factor is any measurement available at the time of surgery that correlates with disease-free or overall survival in the absence of systemic adjuvant therapy and, as a result, is able to correlate with the natural history of the disease. In contrast, a predictive factor is any measurement associated with response to a given therapy. Lymph node status, Tumour size, Lymphovascular invasion, Proliferation markers, Ethnicity, and Age are the prognostic factors while ER/PR status, HER2/neu, Urokinase-type plasminogen activator (uPA) and its inhibitor, plasminogen activator inhibitor type 1 (PAI-1), Genetic profiling are prognostic as well as predictive factor [5]. Angiogenesis is required for invasive tumour growth, metastasis; and constitutes an important role in the control of cancer progression. Its inhibition may be a valuable new approach to cancer therapy [6]. Angiogenesis provide increased availability of oxygen and nutrients to the tumour as well as the most important route of exit from the primary tumour into the blood stream [7]. Assessment of intratumoural microvessel density using immunohistochemistry with panendothelial markers like factor VIII–related antigen, CD31, CD34, and the activated endothelial cell marker CD105 is a commonly used technique for quantifying tumour angiogenesis in breast cancer [8,9]. The presence of estrogen and progesterone receptors in an invasive breast carcinoma is both prognostic and predictive. Its prognostic effect is difficult to evaluate in that it must be assessed in the absence of adjuvant tamoxifen [10]. In the era of targeted therapy, some authors use antiangiogenic therapy to reduce the tumour progression and tumour metastasis. The most important clinical implication of tumour angiogenesis is the development of a novel strategy of anticancer therapy targeting tumour vessels instead of cancer cells. Antiangiogenic therapy aims to inhibit the growth of tumour, and current evidence suggests that it works best in combination with conventional cytotoxic chemotherapy [11]. Evaluation of estrogen and progesterone receptors status is a routine procedure in the management of breast cancer. Receptor status is useful not only for defining prognosis in breast cancer but also for selection of patients for therapy. With this background, the present study was planned to analyse angiogenesis in breast cancer, which can be a potential target of the newer anti-angiogenic drugs to treat and improve the clinical outcome of this disease.
Materials and Methods
The present study included tumour sample from the mastectomy specimens received in the Department of Pathology, Maulana Azad Medical College during the period between Jan 2009 to Jun 2010 of 28 untreated cases of carcinoma breast in an age group of 25-75 y with a mean of 43.5 y. All the cases were evaluated by history, physical and local examination. The paraffin embedded sections were made from sample obtained by representative mastectomy specimen. Histopathological examination included staining of section by haematoxylin & eosin stain and immunohistochemistry by CD34, ER and PR antibody. Histological grading was done according to Elston's modified Bloom and Richardson method [12]. The positive staining was defined as brown granular nuclear staining in > 10% of the cells. The angiogenesis was demonstrated using CD 34 antibody and microvessels density was calculated in an area containing the greatest number of discrete micro vessels at low power followed by high power. Five fields were examined and mean of micro vessel density was calculated. A single countable vessel was defined as any brown staining of any endothelial or endothelial cell cluster separate from adjacent micro vessels, tumour cells or connective tissue elements. The vessel calibre and vessels lumens having erythrocytes were not included in the criteria for defining micro vessels. For statistical analysis, cases were grouped into estrogen, progesterone positive or negative receptors and they were correlated with mean vascular density and prognostic factors.
Results
Out of 28 cases, 24 were infiltrating duct carcinoma, two of metaplastic carcinoma and 1 of mixed lobular and ductal carcinoma and sarcoma was included in the study [Table/Fig-1,2]. 89% of the cases attained early menarche with mean age of 11.24 y and 32% cases attained late menopause after 55 y of age. The presenting complaints were lump (100%); weight loss (57.1%) and nipple discharge (14.28%). The most common quadrant involved was upper outer quadrant (42.85%) followed by central quadrant (39.29%). 62.5% of cases were grade II and 25% grade III rest were grade I. Lymph node metastasis were present in 89% of cases. The patients were grouped into ER-/PR – (39.29%), ER+/PR + (32.14%), ER-/PR+ (21.43%) and ER+/ PR-, (7.14%) respectively. The mean micro vessel density calculated for ER-/PR-, ER-/ PR+, ER+/PR-, ER+/PR+ was 15.45, 14.83, 11, and 10.89. The mean micro vessel density calculated for grade III was 14.17, grade II 12.93 and grade I 12.33. A post– ANOVA comparison test was performed using Bonferonni’s multiple comparison tests [Table/Fig-3]. An insignificant difference was observed in MVD between the four groups (p-value < 0.05) and significant correlation was found between ER receptors and mean vascular density (p value < 0.05) [Table/Fig-4]. The tumour neovasularization was found to be independent of the tumour size, histological grade, necrosis, embolization, adipose tissue infiltration, perinodal extension, morphological types and lymph node metastasis [Table/Fig-5].
a. Infiltartive ductal carinoma NOS Grade I ( H&E x100)
b. Infiltartive ductal carinoma NOS Grade II ( H&E x100)
c. Infiltartive ductal carinoma NOS Grade III ( H&E x100)
d. Lobular carcinoma of breast (H&E x100)

a. Metaplastic carcinoma with squamoid differentiation (H&E x400)
b. Vascular embolisation by tumour cells (H&E x100)
c. ER positivity in Infiltartive ductal carinoma NOS (ER LSAB Peroxidase x400)
d. PR positivity in Infiltartive ductal carinoma NOS (PR LSAB Peroxidase x400)

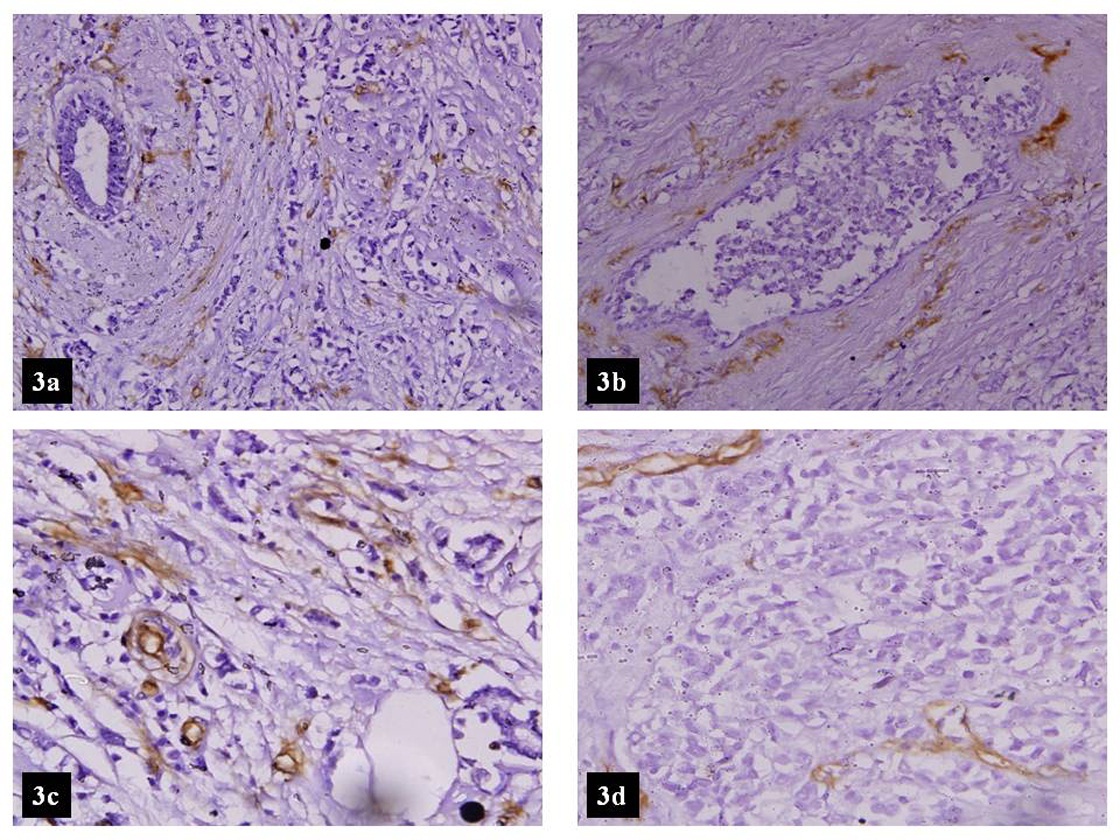
a. High MVD in mixed lobular and ductal ( CD-34 LSAB Peroxidase x100)
b. High MVD around a focus of DCIS ( CD-34 LSAB Peroxidase x100)
c. Tumour with high MVD ( CD-34 LSAB Peroxidase x100)
d. Tumour with low MVD (CD-34 LSAB Peroxidase x100)
Association between ER/PR status and the micro vessel density using, a post – anova comparison test by using bonferonni’s multiple comparison tests
| Status | p- value |
|---|
| ER | <0.05 |
| PR | >0.05 |
A significant correlation is found between ER receptors and mean vascular density [p value (< 0.05)]
Association between various variables and the microvessel density
| Variable | | No of Cases | Mean MVDαStandard Deviation | p- value |
|---|
| Tumour Size | < 3 cm | 15 | 12.53α3.50 | >0.05 |
| > 3 cm | 13 | 12.63α3.37 |
| Histological Grade | I | 03 | 12.33α4.92 | >0.05 |
| II | 15 | 12.93α3.26 |
| III | 06 | 14.17α0.98 |
| Necrosis | Absent | 21 | 13.47α3.04 | >0.05 |
| Present | 07 | 13.71α3.09 |
| Embolization | Absent | 21 | 14.047α2.59 | >0.05 |
| Present | 07 | 12.00α3.78 |
| Adipose Tissue Infiltration | Absent | 19 | 14.05α2.95 | >0.05 |
| Present | 09 | 12.44α2.96 |
| Perinodal Extension | Absent | 16 | 13.75α3.13 | >0.05 |
| Present | 12 | 13.252.92 |
| Metastastic Lymph Node | | 25 | | >0.05 |
| Morphological Types | Infiltrating duct carcinoma | 24 | 15.75α3.03 | >0.05 |
| Others | 04 | 13.16α1.07 |
Discussion
In the last few decades, targeted therapies gained the importance to treat the cancer and prevent their progression and metastasis. Angiogenesis is an important component of cancer growth, invasion and metastasis. Therefore, inhibition of angiogenesis is an attractive strategy for treatment of cancer [13]. In our study, all cases were grouped as ER-/PR-, ER-/ PR+, ER+/PR-, ER+/PR+ for statistical analysis. Vamesu studied the 158 needle core biopsy for angiogenesis, ER/PR status in primary breast cancer patients and observed that the highest mean vascular density was seen in ER-/ PR- individuals followed by ER+ / PR+ [14]. In our study highest mean vascular density was observed in ER-/ PR- followed by ER-/ PR+ and ER+ / PR-. The variation in the value of mean MVD could be attributed to the different methodologies, particularly the measurement of MVD, definition of controls and number of subjects included in the study. Biesaga also observed significant correlation between MVD and ER negative and PR negative tumours [15]. Parentes JB et al., observed that the mean number of vessels stained with anti cd34 antibody in the estrogen receptors positive and negative tumours was 23.51 and 40.24. The number of micro vessel was significantly greater in the estrogen receptors negative tumours. Parentes JB et al., concluded that ER negative tumours have significantly greater CD34 antigen expression compared to ER positive tumours [16]. Dhakal et al., also observed a significant correlation (p< 0.003) with mean vascular count and estrogen and progesterone negativity [17]. In the current study a significant correlation was p value (< 0. 05) seen with estrogen receptors status and mean vascular density however no such significant correlation was seen with progesterone receptors. It was also observed that there was an increase in mean vascular density of tumour from histological grade I to grade III. However, no such correlation of mean vessel counts with histological grades (p == 0.63) was observed which was similar to the findings observed by Biesaga et al., [15], Fisher et al., [18] and Miliaras et al., [19] while Dhakal et al., [17] observed a significant correlation with mean vascular count and histological grade. Miliaras et al., [19], Iochaim et al., [20] and Biesaga et al., [15] observed no association of mean vascular density with tumour size which was similar to that observed in the present study (p=0.94), but Gasparini et al., [21] founded a significant correlation with tumour size. In the present study there was no significant association seen with morphological type (p=0.112), adipose tissue infiltration (p=0.120), embolization (p=0.120), necrosis (p=0.860), and perinodal extension (p=0.671). Similar finding was observed in study done by Gasparini et al., [21]. Nodal metastasis was also found to be independent of the tumour vascularity (p=0.898).
Conclusion
ER negative tumours showed greater CD34 antigen expression. While the tumour neovasularization was found to be independent of the tumour size, histological grade, necrosis, embolization, adipose tissue infiltration, perinodal extension, morphological types and lymph node metastasis. We would also emphasize that the quantitative determination of micro vessel density may be important, not only for prognostic value, but also because it may help to predict responses to angiogenesis inhibiting drugs.
A significant correlation is found between ER receptors and mean vascular density [p value (< 0.05)]
[1]. Khokhar A, Breast Cancer in India: Where Do We Stand and Where Do We Go?Asian Pacific Journal of Cancer Prevention 2012 13:4861-66. [Google Scholar]
[2]. Agarwal G, Ramakant P, Breast Cancer Care in India: The current scenario & the challenges for futureBreast Care (Basel) 2008 3:21-27. [Google Scholar]
[3]. Green M, Raina V, Epidemiology, screening and diagnosis of breast cancer in the Asia–Pacific region: current perspectives and important considerationsAsia Pacific J Clin Oncol 2008 4(3):5-13. [Google Scholar]
[4]. Leong SPL, Shen ZZ, Liu TJ, Agarwal G, Tajima T, Paik NS, Is Breast Cancer the Same Disease in Asian and Western Countries?World J Surg 2010 34:2308-24. [Google Scholar]
[5]. Cianfrocca M, Goldstein LJ, Prognostic and predictive factors in early-stage breast cancerThe Oncologist 2004 9:606-16. [Google Scholar]
[6]. Folkman J, Role of angiogenesis in tumour growth and metastasisSemin Oncol 2002 29:15-18. [Google Scholar]
[7]. Bruce R, Zetter. Angiogenesis and tumour metastasisAnnu. Rev Med 1998 49:407-24. [Google Scholar]
[8]. Uzzan B, Nicolas P, Cucherat M, Perret GY, Microvessel density as a prognostic factor in women with breast cancer: a systematic review of the literature and meta-analysisCancer Res 2004 64:2941-55. [Google Scholar]
[9]. Fox SB, Harris AL, Histological quantifcation of tumour angiogenesisAPMIS 2004 112:413-30. [Google Scholar]
[10]. Fisher B, Redmond C, Fisher ER, Relative worth of estrogen or progesterone receptor and pathologic characteristics of differentiation as indicators of prognosis in node-negative breast cancer patients. Findings from National Surgical Adjuvant Breast and Bowel Project Protocol B-06J Clin Oncol 1988 6:1076-87. [Google Scholar]
[11]. Pang RWC, Poon RTP, Clinical implications of angiogenesis in cancersVascular health and risk management 2006 2:97-108. [Google Scholar]
[12]. Frierson Jr HF, Wolber RA, Berean KW, Franquemont DW, Gaffey MJ, Boyd JC, Wilbur DC, Interobserver reproducibility of the Nottingham modification of the Bloom and Richardson histologic grading scheme for infiltrating ductal carcinomaAm J Clin Pathol 1995 103:195-98. [Google Scholar]
[13]. Nielsen DL, Andersson M, Andersen JL, Kamby C, Antiangiogenic therapy for breast cancerBreast Cancer Research 2010 12:209 [Google Scholar]
[14]. Vamseu S, Angiogenesis and Estrogen and Progesterone status in primary breast cancer patient: an analysis of 158 needle core biopsiesRom J Morphol Embryol 2007 48:25-31. [Google Scholar]
[15]. Biesaga B, Joanna Niemiec J, Ziobro M, Microvessel density and status of p53 protein as potential prognostic factors for adjuvant anthracycline chemotherapy in retrospective analysis of early breast cancer patients groupPathol Oncol Res 2012 18:949-60. [Google Scholar]
[16]. Parentes-Vieiera JB, Lopes-Costa PV, Pires CG, Santos AR dos, Pereira-Filho JD, da Silva BB, Quantification of angiogenesis in estrogen positive receptors and negative carcinomaInternational seminar in surgical oncology 2007 4:22 [Google Scholar]
[17]. Dhakal HP, Naume A, Synnestvedt M, Borgen E, Kaareseen R, Schlichting E, Vascularization in primary breast carcinoma. Its prognostic significance and relationship with tumour cell disseminationClinic Cancer Res 2008 14:2341-50. [Google Scholar]
[18]. Fisher ER, Costantino J, Fisher B, Redmond C, Pathological finding from the National surgical adjuvant breast projectCancer 1993 68:2142-50. [Google Scholar]
[19]. Miliaras D, Kamas A, Kalekou H, Angiogenesis in invasive breast carcinoma: it is associated with parameters of prognostic significance?Histopathology 1995 26:165-69. [Google Scholar]
[20]. Ioachim E, Charchanti A, Charalabopoulos K, Tsanou H, Briasoulis E, Karavasilis V, The prognostic evaluation of tumour angiogenesis in invasive breast carcinomaElectronic Journal of Pathology and Histology 2002 8:21-22. [Google Scholar]
[21]. Gasparini G, Weidner N, Bevilacqua P, Maluta S, Palma DP, Caffo O, Tumour Microvessel density, p53expression, Tumour size and Peritumoural lymphatic invasion are relevant prognostic markers in node negative breast carcinomaJ Clin Oncol 1994 12(3):454-66. [Google Scholar]