ALK Positive Anaplastic Large Cell Lymphoma Presenting as Extensive Bone Involvement
Smeeta Gajendra1, Ritesh Sachdev2, Lipika Lipi3, Shalini Goel4, Ruchira Misra5
1 Attending Consultant, Department of Pathology and Laboratory Medicine, Medanta-The Medicity, Sector –38, Gurgaon, Haryana, India.
2 Senior Consultant, Department of Pathology and Laboratory Medicine, Medanta-The Medicity, Sector –38, Gurgaon, Haryana, India.
3 Associate Consultant, Department of Pathology and Laboratory Medicine, Medanta-The Medicity, Sector –38, Gurgaon, Haryana, India.
4 Attending Consultant, Department of Pathology and Laboratory Medicine, Medanta-The Medicity, Sector –38, Gurgaon, Haryana, India.
5 Consultant, Department of Pediatric Medical Oncology, Medanta-The Medicity, Sector –38, Gurgaon, Haryana, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Ritesh Sachdev, Senior Consultant, Department of Pathology and Laboratory Medicine, Medanta-The Medicity, Sector-38, Gurgaon, Haryana 122 001, India.
E-mail: sachdev05@gmail.com
Anaplastic lymphoma kinase (ALK) positive Anaplastic large cell lymphoma (ALCL) represents approximately 2% of all Non-Hodgkin’s lymphomas that commonly involves nodal as well as a wide variety of extra nodal sites, as skin, soft tissue, bones and lungs, although primary or secondary involvement of bone is rare. Herein, we report a case of 14-year-old female child presented as extensive bony involvement with a clinical diagnosis of bone tumour/ small round cell tumour, which was proved to be ALK positive ALCL on histopathological examination.
Anaplastic large cell lymphoma, Anaplastic lymphoma kinase, CD30, Multifocal osseous involvement
Case Report
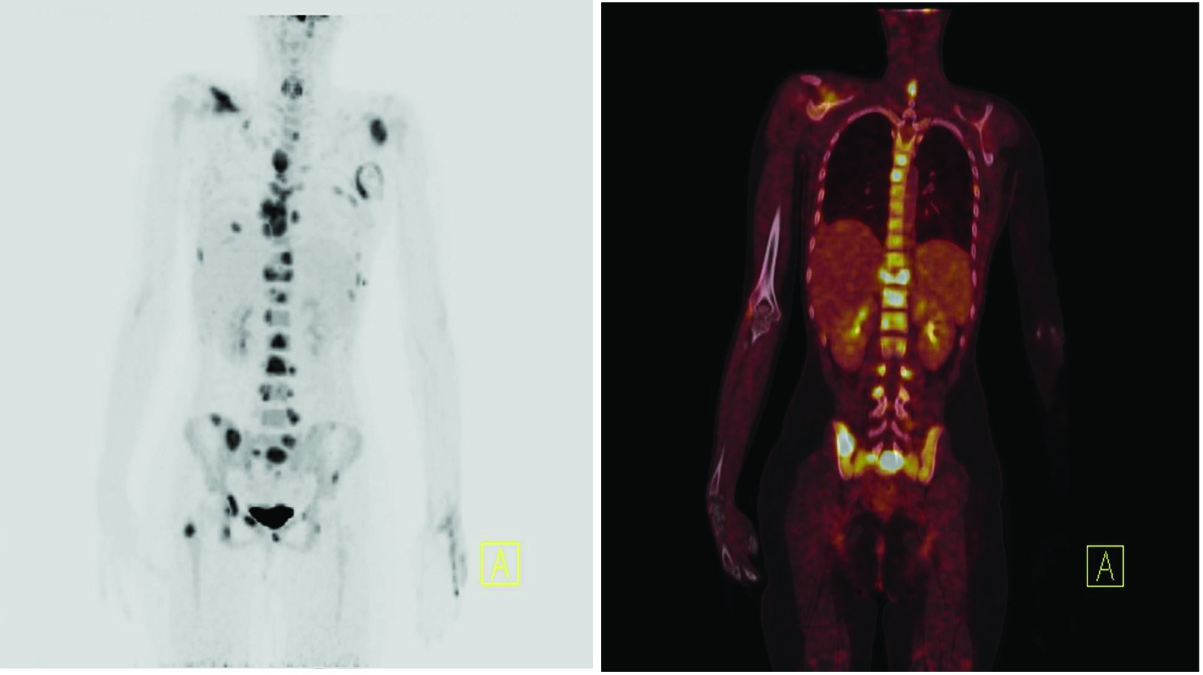
A 14-year-old female child came to us with left sided chest pain, weight loss and difficulty in breathing since two months. On examination, she had left axillary lymphadenopathy, no hepatomegaly, mild splenomegaly (just palpable below costal margin) and skin lesion on left chest wall. Complete blood counts, liver function tests, renal function tests and Lactate dehydrogenase (LDH) were within normal limit. 18F FDG PET/CT (Biograph m CT, SIEMENS) showed expansile lytic lesions with increased FDG avidity involving the clivus on left side, bilateral scapula, sternum with associated soft tissue, multiple ribs, multiple vertebrae with compression fracture of D11, bilateral iliac bones, bilateral ischium, sacrum, right superior and inferior pubic rami, bilateral acetabulum and right femoral neck [Table/Fig-1]. There was hypermetabolic subcutaneous soft tissue deposit in the left lateral chest wall. Based on extensive bony involvement and normal LDH, a clinical diagnosis of bone tumour/ small round cell tumours was considered. But, axillary lymph node biopsy showed effacement of lymph node architecture by sheets of large atypical pleomorphic cells with prominent nucleoli and brisk mitosis [Table/Fig-2]. Immunohistochemically, these cells showed positivity for CD45 (heterogenous), CD30(Dako; Ber-H2), CD5(Dako; SP 19), CD43, Anaplastic lymphoma kinase (ALK) (Dako; CD246 ALK1), Epithelial membrane antigen(EMA) (Dako; E20), and Ki 67(Dako; MIB-1) (80%) ; and negativity for CD3(IS 503), CD56(Dako; 123C3), CD20(Dako; L26),CD10(Dako; 56C6), PAX5(Biogenix; ZP007) and CD138 (Dako; MI 15) [Table/Fig-3]. A diagnosis of ALK positive ALCL was made. Bone marrow examination and Cerebrospinal fluid cytology done for staging showed no morphological evidence of lymphoma infiltration. The patient was started on combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) is started. Radiation therapy to bulky sites of disease was planned after completion of chemotherapy.
18F FDG PET/CT showing expansile lytic lesions with increased FDG avidity involving multiple bones
Histopathological microphotograph (hematoxylin and eosin, 20x and 40x) showing large blastic cells with eccentric nuclei and abundant cytoplasm

Immunohistochemistry of ALCL showing expression of CD30, ALK, CD5, EMA, Ki 67 -80% but no expression of CD3, CD20 or CD15

Discussion
ALCL is a rare but well characterised lymphoma, occurring in approximately 2% of all Non-Hodgkin’s lymphomas, which was first described as a clinical entity in 1985 based on proliferation of large pleomorphic cells expressing CD 30 [1]. It commonly involves nodal as well as a wide variety of extra nodal sites, as skin, soft tissue, bones and lungs, although primary or secondary involvement of bone is rare [2]. ALCL was first included in the World Health Organization (WHO) classification of lymphoid neoplasms in 2001. The current WHO classification (2008) describes two entities of ALCL according to ALK protein expression in tumour samples: (a) a distinct entity, named ALK+ ALCL, which is characterized ALK protein expression; and (b) a provisional entity, the so-called ALK– ALCL, which cannot be distinguished morphologically from ALK+ ALCL but differs from this entity because of the lack of ALK protein [3]. There is a male predominance, particularly in ALK+ cases, in which the male/female ratio is approximately 3:1. Translocation (2;5) involving ALK gene on chromosome 2p23 and the nucleophosmin gene (NPM) on chromosome 5q35occurs in most case of ALCL. Unscheduled expression of the truncated ALK contributes to malignant transformation in these lymphomas [4]. ALK+ALCL and ALK- ALCL have different gene-expression profiles also. Among the most significantly differentially expressed genes between ALK+ and ALK- samples, BCL6, PTPN12, CEBPB, and SERPINA1 genes are overexpressed in ALK+ ALCL, where as in ALK- ALCL, there is overexpression of CCR7, CNTFR, IL22, and IL21 genes [5]. On histopathology, the “hallmark” or classic ALCL cell contains an eccentric nucleus that is generally horseshoe shaped or reniform with prominent nucleoli, abundant cytoplasm and prominent eosinophilic golgi region [6]. It may present as diagnostic challenge for the pathologist and clinician, but the advances in immunophenotyping and cytogenetic characterization however, have largely eliminated much of the diagnostic confusion [7]. Immunophenotypically, ALCL cells are commonly positive for CD45, HLA-DR, CD25, CD30, while CD15 is most often negative. In about 60% of ALCL, there is expression of one or more T-cell associated antigens, such as CD3, CD43, or CD45RO. In few cases of ALCL, there is neither B nor T cell antigen expression, which is designated as null cell type [8]. Non-Hodgkin’s lymphomas uncommonly present as bone lesions. Most of these tumours are diffuse large B-cell lymphomas. ALCL presenting as extensive bony lesions at the time of diagnosis is uncommon. Therefore, lymphoma involving bone should be considered in the differential diagnosis. Radiological bone imaging revealed nonspecific lytic lesions while increased tracer uptake seen on radionuclide scan. Adequate biopsy sample for histopathology, immunotyping and immunohistochemistry is critical for definitive diagnosis of ALCL.
Conclusion
ALCL with extensive bone involvement is rare at initial presentation. As in our case, this may be misdiagnosed as small round cell bone tumour. Therefore, from the clinical and radiological point of view, lymphoma involving bone should be considered in the differential diagnosis of a young adult presenting with multifocal osseous involvement that help in prompt treatment and improves survival of the patients.
[1]. Nagasaka T, Nakamura S, Medeiros LJ, Juco J, Lai R, Anaplastic large cell lymphomas presented as bone lesions: a clinicopathologic study of six cases and review of the literatureMod Pathol 2000 13(10):1143-49. [Google Scholar]
[2]. Rahmat K, Wastie M, Abdullah B, Primary bone lymphoma: report of a case with multifocal skeletal involvementBiomed Imaging Interv J 2007 3(4):e52 [Google Scholar]
[3]. Mason DY, Harris NL, Delsol G, in World Health Organization Classification of Tumours: Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues, Anaplastic large cell lymphoma, ALK-negative, eds Swerdlow S, Campo E, Harris NL, et al 2008 Lyon, FranceIARC Press:317-19. [Google Scholar]
[4]. Eyre TA, Khan D, Hall GW, Collins GP, Anaplastic lymphoma kinase-positive anaplastic large cell lymphoma: current and future perspectives in adult andpaediatric diseaseEur J Haematol 2014 doi: 10.1111/ejh.12360 [Google Scholar]
[5]. Lamant L, de Reyniès A, Duplantier MM, Rickman DS, Sabourdy F, Giuriato S, Gene-expression profiling of systemic anaplastic large-cell lymphoma reveals differences based on ALK status and two distinct morphologic ALK+ subtypesBlood 2007 109(5):2156-64. [Google Scholar]
[6]. Wright D, McKeever P, Carter R, Childhood non-Hodgkin lymphomas in the United Kingdom:findings from the UK Children’s Cancer Study GroupJ Clin Pathol 1997 50:128-34. [Google Scholar]
[7]. Jacobsen E, Anaplastic Large-Cell Lymphoma, T-/Null-Cell TypeThe Oncologist 2006 11:831-40. [Google Scholar]
[8]. Jaffe ES, Krenacs L, Raffeld M, Classification of cytotoxic T-cell and natural killer cell lymphomasSemin Hematol 2003 40:175-84. [Google Scholar]