Large Congenital Nevus Scalp Managed by Tissue Expansion– A Case Report
Surya Rao Rao Venkata Mahipathy1, Alagar Raja Durairaj2
1Assistant Professor, Department of Plastic Surgery, Saveetha Medical College Hospital, Thandalam, Kanchipuram, India.
2Assistant Professor, Department of Plastic Surgery, Saveetha Medical College Hospital, Thandalam, Kanchipuram, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Surya Rao Rao Venkata Mahipathy, Assistant Professor, Department of Plastic Surgery, Saveetha Medical College Hospital, Thandalam, Kanchipuram- 602105, India.
E-mail: surya_3@hotmail.com
Tissue expansion is an emerging trend in the reconstruction of various defects of the human body when there is insufficient adjacent tissue for direct closure of the defect or repair with a local tissue transfer. It is the most important armamentarium for aesthetic hair-bearing scalp reconstruction in cases of congenital or acquired defects. In this case, a large linear verrucous epidermal nevus is excised and covered with expanded hair-bearing scalp tissue.
Hair-bearing scalp, Nevus, Tissue expansion
Case Report
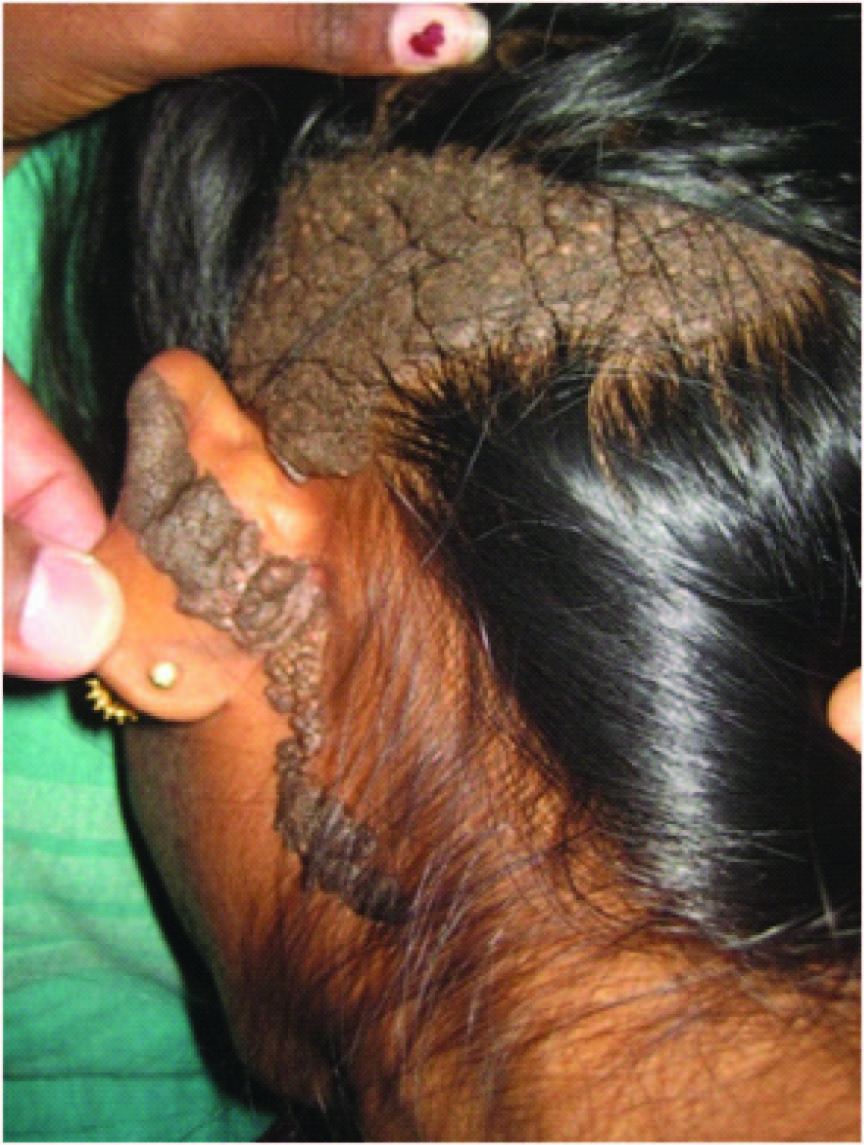
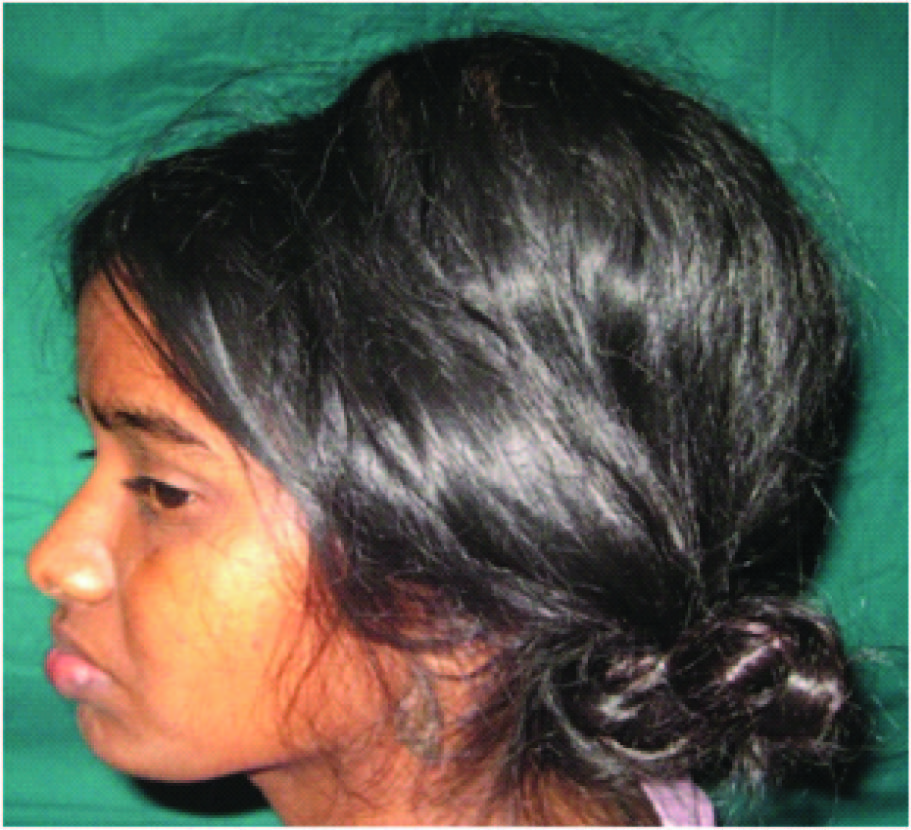
An-18-year-old girl presented to us with a large nevus on the left side of the scalp and left postauricular region since birth. Initially it was small but gradually increased present size. There was no history of pain, bleeding, pruritus from the nevus. No other similar lesions were found elsewhere. Dermatology opinion was sought and it was diagnosed as linear verrucous epidermal nevus. On examination, 10cm x 5cm x 1cm black, warty verrucous lesion was present involving the temporooccipital region [Table/Fig-1] . Lesion was firm, non-tender and not friable. There was no regional lymphadenopathy. The estimated defect size post excision was 11 x 6 x 1.5cm.
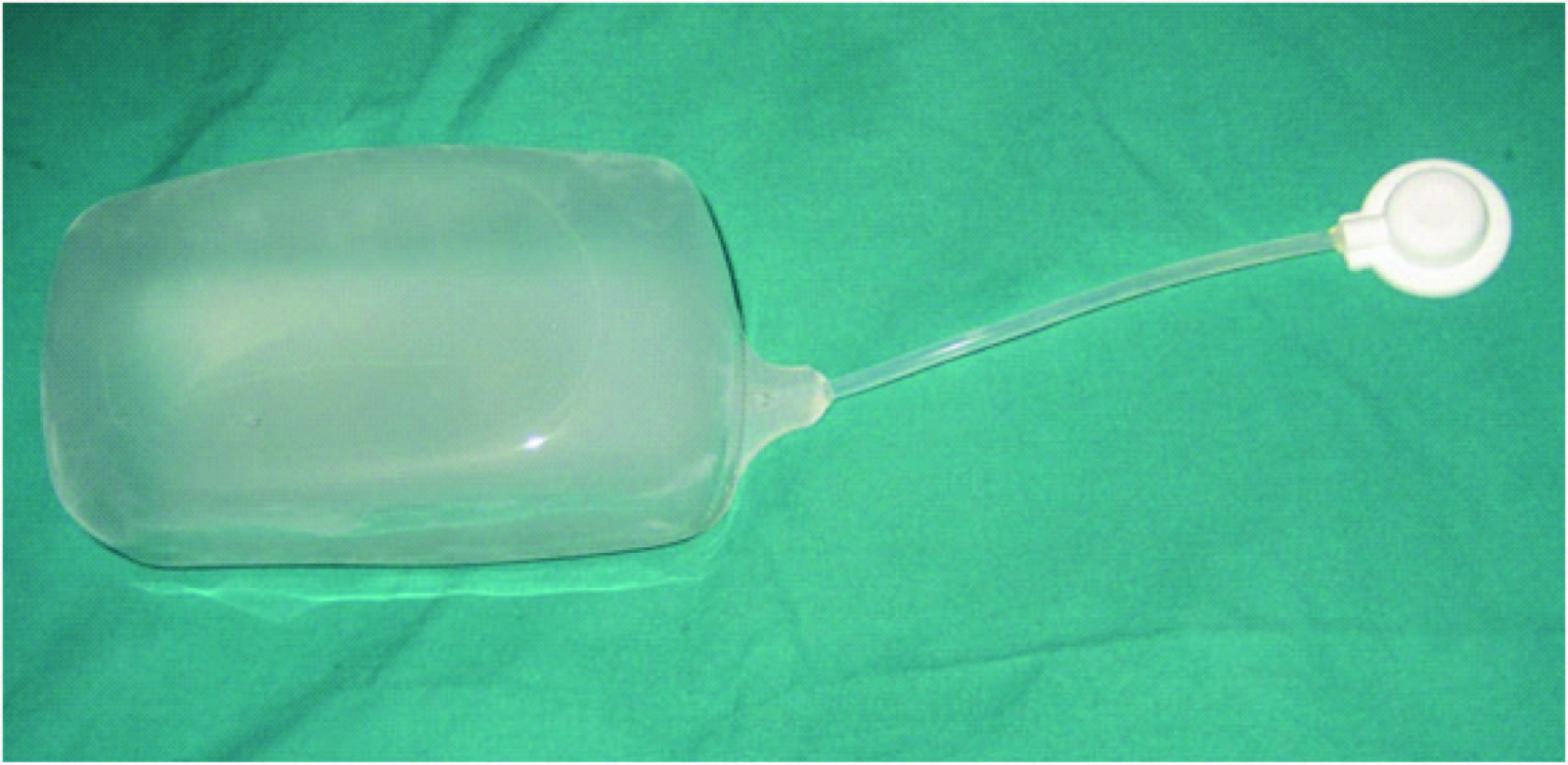
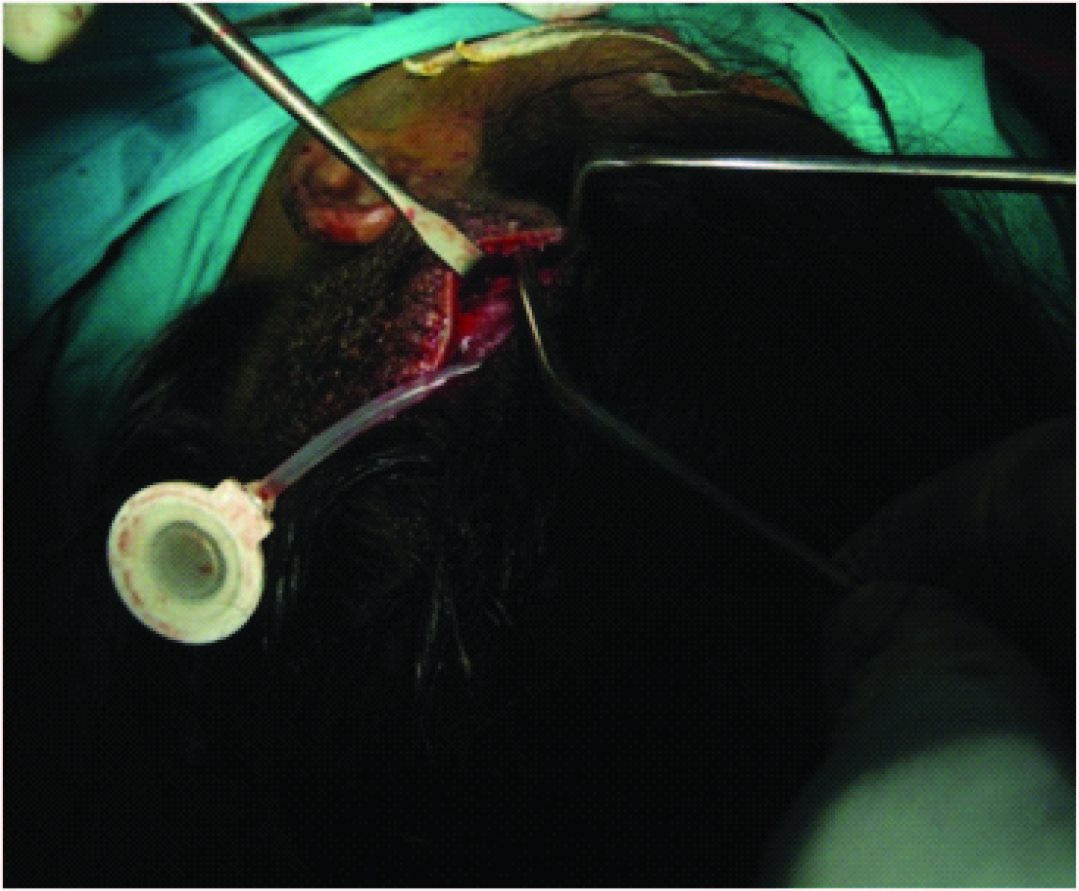
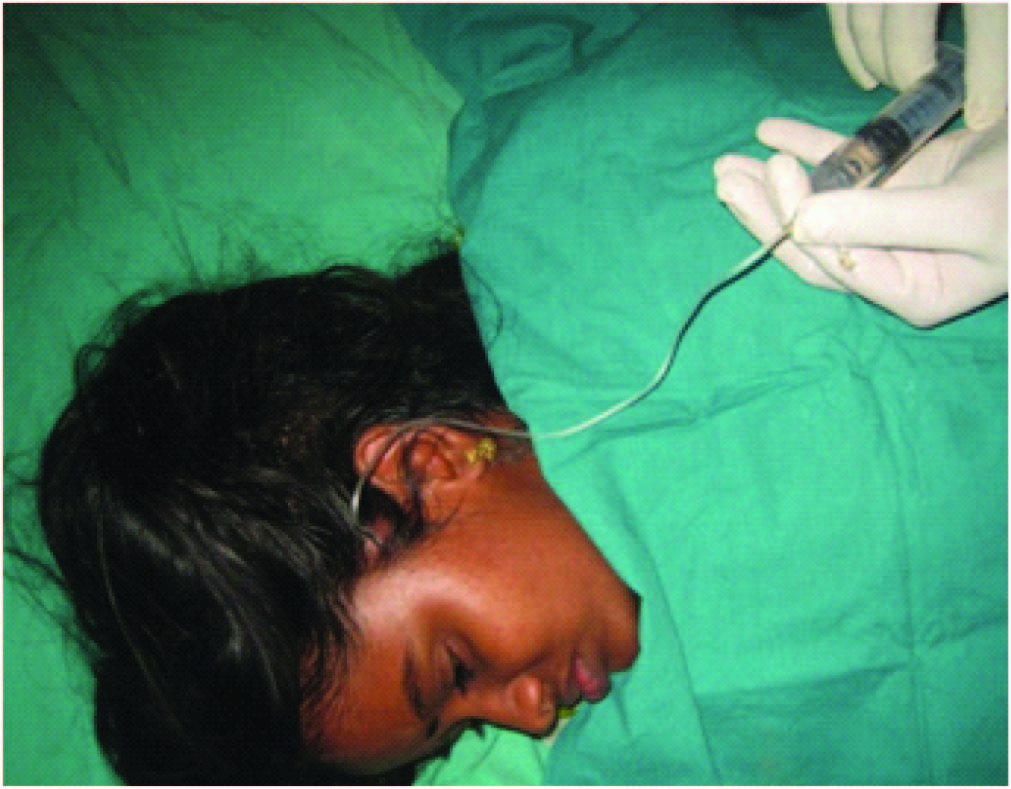
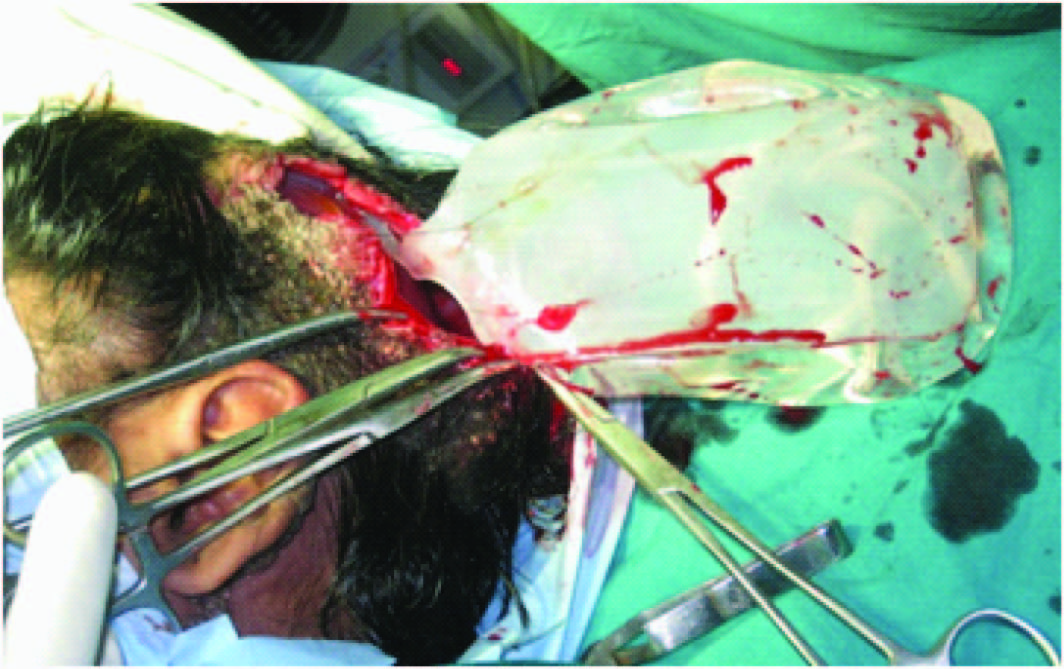
The plan was excision of the nevus with skin cover. The options were split skin graft, free tissue transfer and tissue expansion. The patient opted for tissue expansion as split skin graft would cause alopecia and there was no adequate hair-bearing scalp to use a free flap. Under general anaesthesia, an 5cm incision was made 1cm adjacent to the lesion on the parietal scalp and a subgaleal plane was developed. A 550ml rectangular silicon expander with remote port was inserted with the remote port placed in a subcutaneous plane in the pre-auricular region and the incision closed [Table/Fig-2,3] . Suture removal was done on 14th post-operative day and expansion was started on 21st day, about 80ml saline every week done for 1.5 months making a total expansion volume of 520ml. This was done as an outpatient procedure using aseptic precautions. A No.24 scalp vein set and a 20ml syringe was used to inject saline into the remote port [Table/Fig-4,5] .
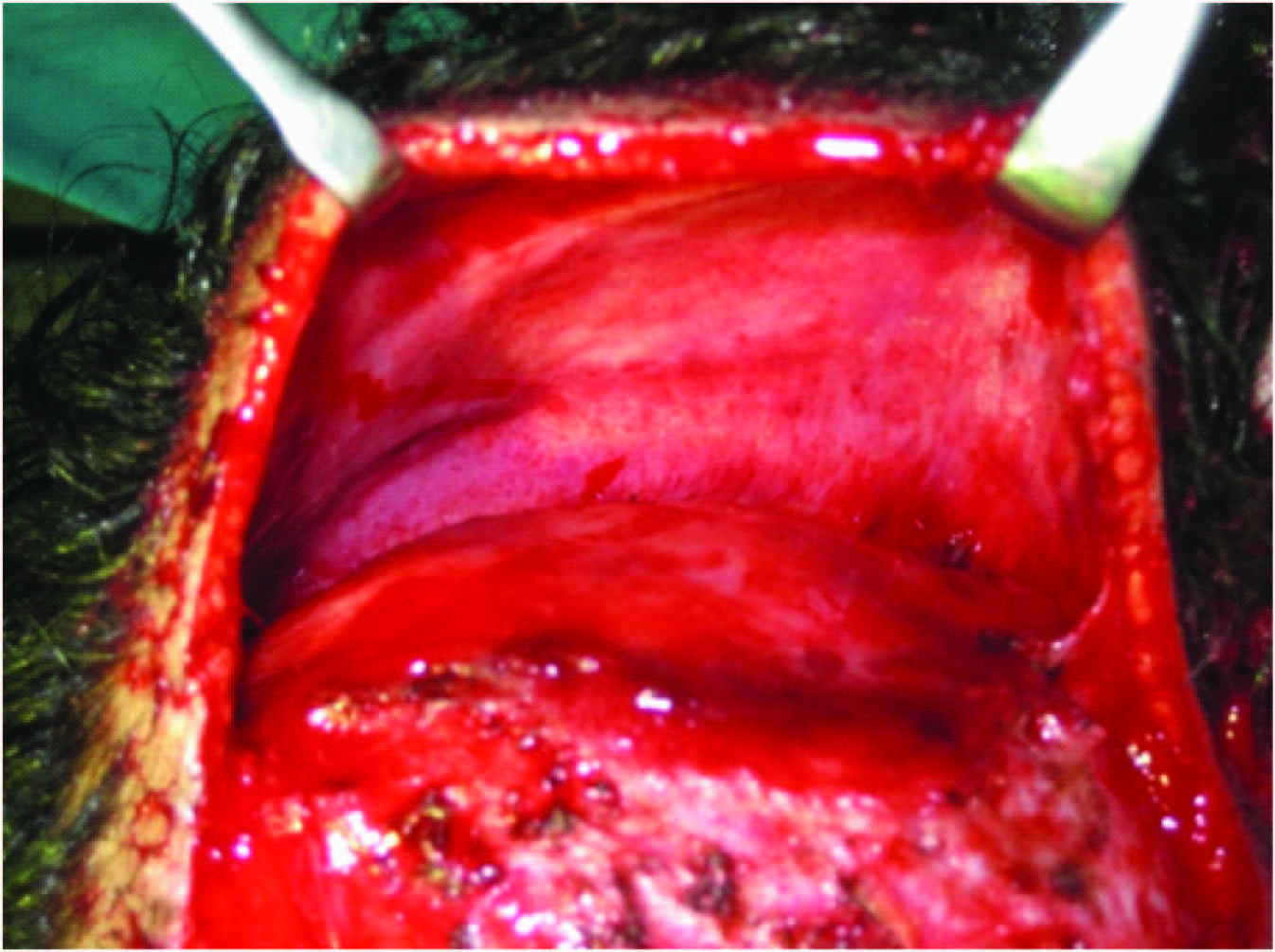
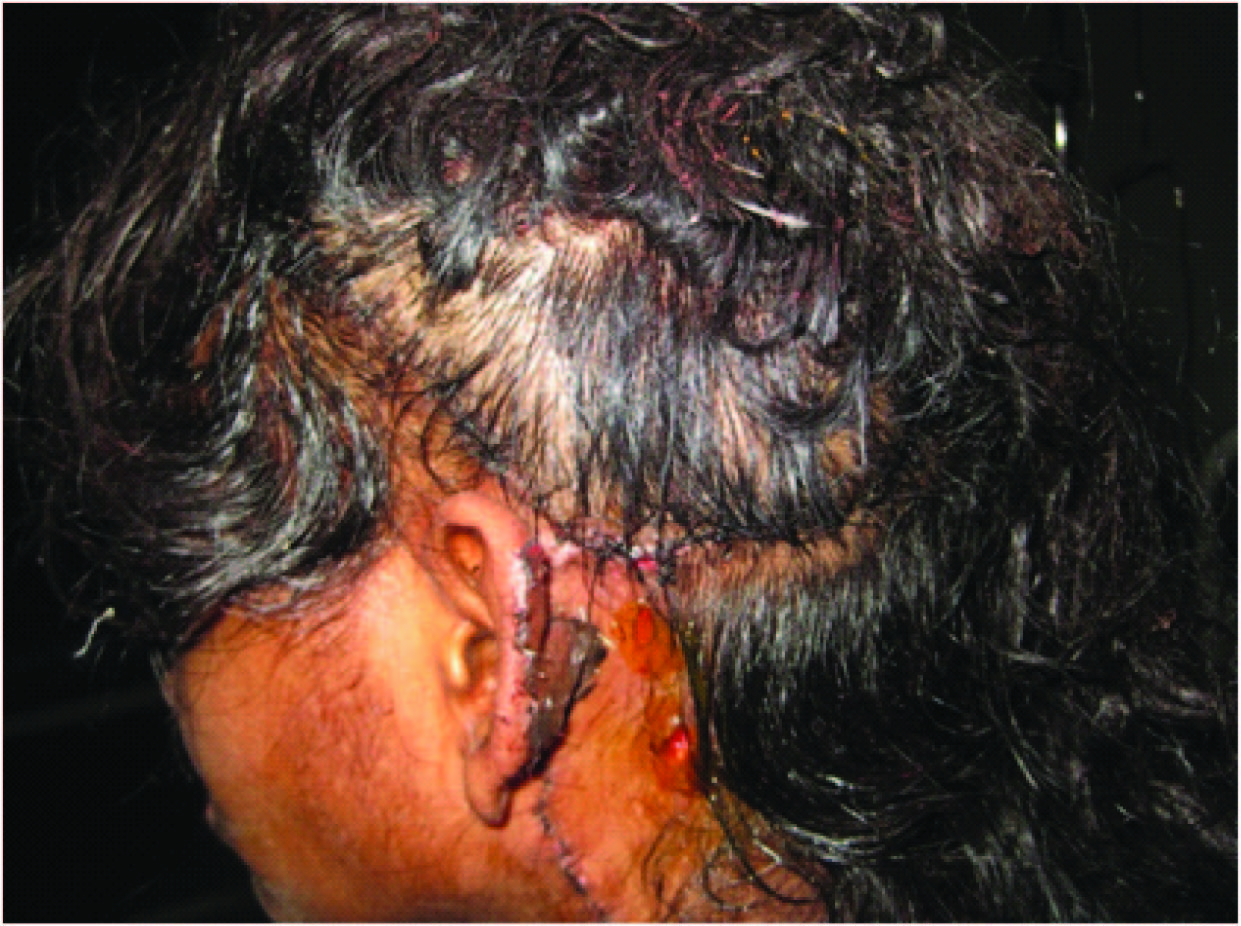
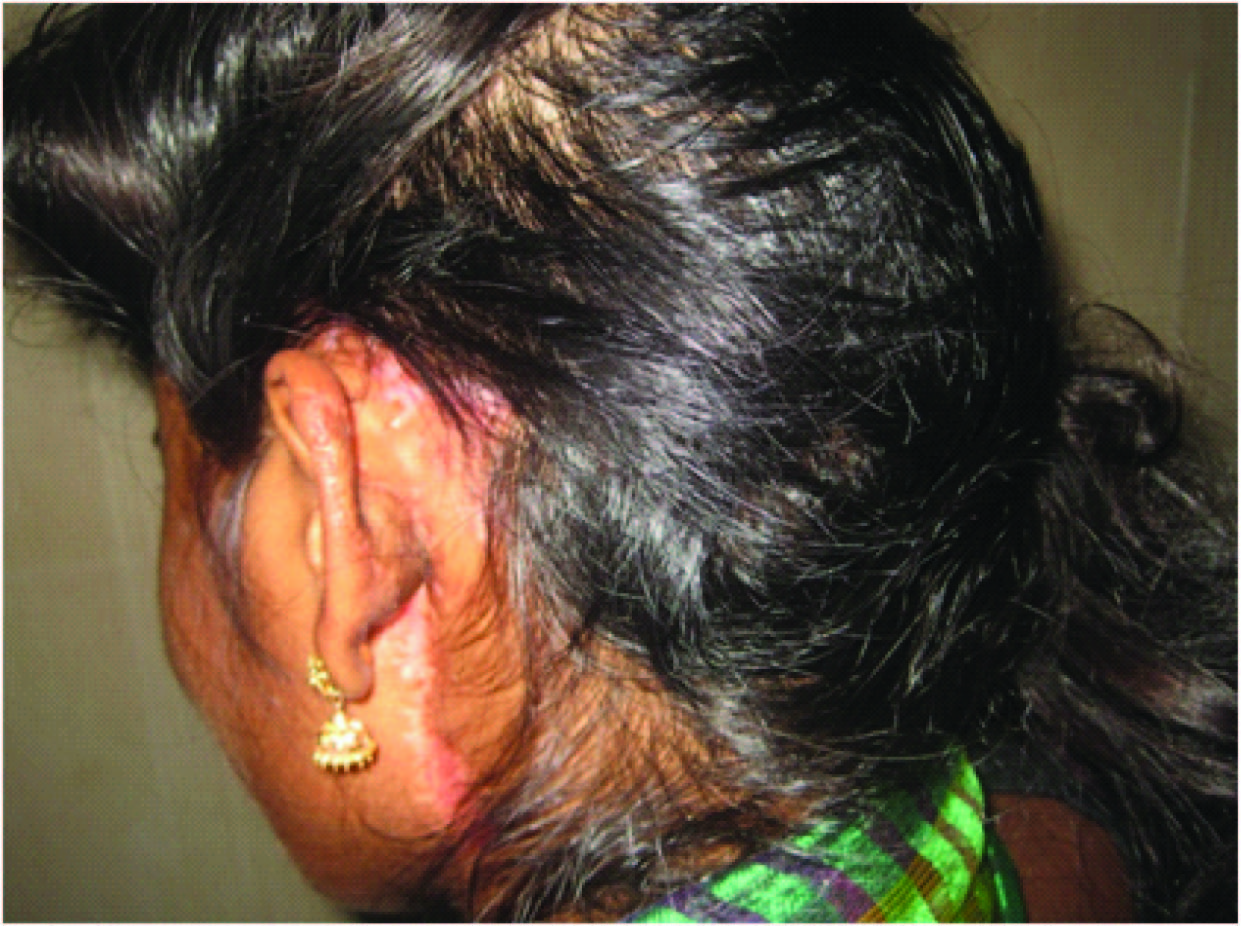
Ten weeks post expander insertion, the expander was removed and the nevus was excised under general anaesthesia [Table/Fig-6,7] . Hemostasis was secured and the defect was covered with the advancement of the expanded scalp flap [Table/Fig-8] . The post-auricular nevus was also excised and closed primarily and partly covered with split skin graft [Table/Fig-9] . Post-operative status was uneventful, sutures were removed on the 10th post-op day and the patient discharged. Histopathology revealed linear epidermal nevus. After surgery, the patient was followed up for 4 months. Patient was reviewed once a week for initial 2 weeks thereafter once a fortnight for the next month and on a monthly basis later [Table/Fig-10] .
Discussion
The concept of tissue expansion occurs under both natural conditions (i.e., pregnancy and breast development) and abnormal circumstances (i.e., tumour growth). As part of the exotic aesthetics of various cultures, tissue expansion has long been noted as a method to document the positions of social hierarchy [1]. The first clinical case of pure soft tissue expansion was reported by Neumann when he reconstructed the upper two-thirds of the right ear in a 52-year-old man by inserting a rubber balloon above the ear. This expanded flap was then advanced to cover the cartilaginous framework. However, the concept of soft tissue expansion to correct traumatic defects did not become popular for another 20 years. In 1975, both Radovan and Austad’s group began pioneering work on soft tissue expansion [2,3]. Experimental data on the biology of tissue expansion has largely come from animal data. Although it is clear that Radovan became the first surgeon to gain extensive experience with tissue expanders, Austad and colleagues first reported the laboratory experience with expanders prior to clinical use [3].
The basic principles used here are the inherent elasticity of the skin or stress relaxation, mechanical creep and biological creep. There are changes occurring at every layer of the skin. There is epidermal thickening [3,4] thinning of the reticular dermis with increased collagen but with unchanged hair follicles [5,6], decrease in the thickness of the adipose layer [4,5], and increased vascularity of the expanded flap [7]. A highly vascular fibrous capsule of fibroblasts, myofibroblasts & thick collagen bundles is formed parallel to the expander surface.
Expanders
Expanders can be standard, customised, anatomic to the donor site (breast), or differential in fill volume to provide tapering of tissue. In terms of shape, they follow three basic patterns: round, rectangular and crescentic. Expander volumes can range from 50cm3 to 2000cm3.
Expansion Rates
The rate at which expansion occurs postoperatively varies. Expansion has been recommended anywhere from 1 week to 2½ weeks after placement. Injection fractions vary, but 10% of the volume per week is required to complete expansion within a 3-month period. However, individual variations exist and one must be aware of pressure changes that occur with expansion so that flap ischemia and subsequent exposure of the expander do not become problems. Pietila et al., addressed the issue of accelerated expansion with the “overfilling” technique [8]. Overexpansion was considered when expansion was done to the point where dermal capillary flow is zero by laser Doppler flow meter and patient discomfort was high.
Techniques in Tissue Expansion
Intra-operative considerations: Generally, expanders are placed in the subgaleal plane. Meticulous hemostasis is vital. Checking the expander for leakage is an important part of the procedure. The injection of air with the submersion of the expander in saline is reliable for detecting leaks. Alternatively, the use of methylene blue helps to identify expander leaks prior to insertion [9].
Operative techniques: Once the required expansion volume has been reached, advancement or rotation of the expanded flap is usually done. Hudson feels that the best method for maximizing the use of expanded tissue in both a vertical and horizontal direction is to add back cuts to the sides as well as the base of the flap. Scoring the capsule to increase flap advancement has been touted by several authors.
Clinical applications: Expanders are used in scalp for large congenital naevi, burns, cicatricial alopecia, male pattern baldness act as an adjunct to craniofacial reconstruction. In breast, they are used post mastectomy and in aplasia or hypoplasia (Poland’s syndrome). In the trunk region, they are used for giant naevi, vascular malformations and contour defects. They are also used in extremities for covering amputated stump and in syndactyly.
Complications of tissue expansion: In 1984, Manders et al., detailed their experience with 41 expanders in 35 patients as a multisite review [10]. They defined complications as major or minor. Major complications of expansion were those that interrupted the expansion process. They include infection, expander exposure and implant failure. Minor complications were defined as problems that were resolved without failure of the procedure. They are pain, seroma, dog-ears and widening of scar.
Linear verrucous epidermal nevus scalp and post-auricular region measuring 10 x 5 x 1cm
Rectangular silicon expander (550 ml) with remote port
Expander placed in the subgaleal plane
Saline being injected into the expander (conventional expansion)
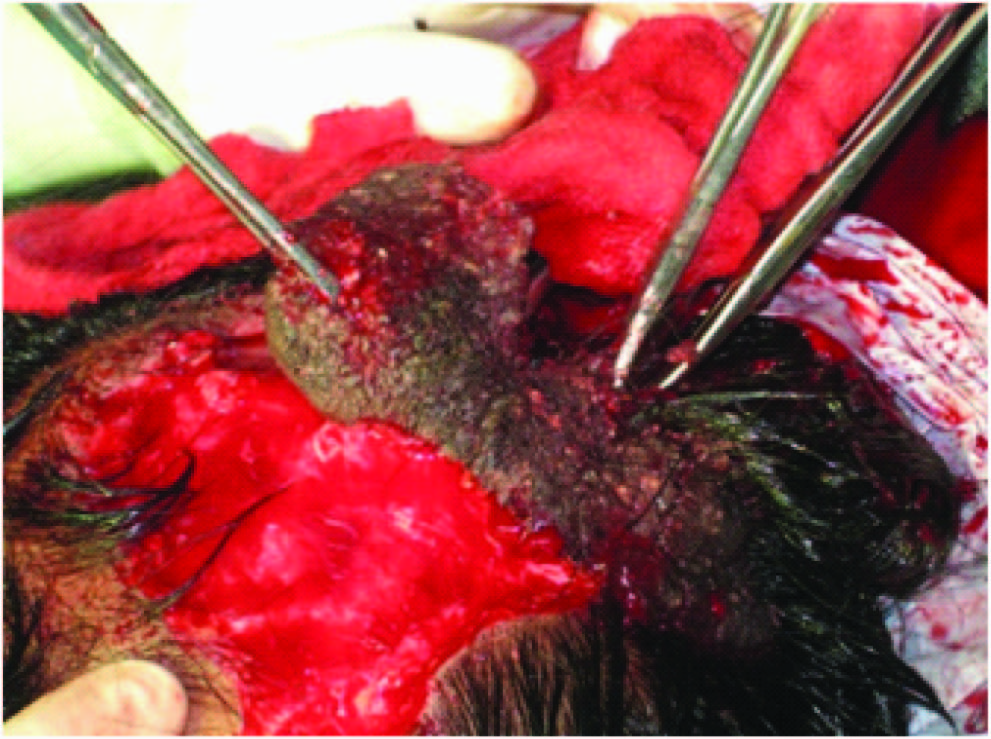
Expanded scalp tissue showing the capsule (Black Arrow)
Immediate post-operative picture
Three months follow-up picture
Conclusion
Reconstruction of soft tissue by tissue expansion has been gaining popularity, especially of the hair bearing scalp. It is superseding other methods of reconstruction of the reconstructive ladder in providing identical tissue with no or minimal morbidity. Even though the process is long, the end results are gratifying for both the patient and the surgeon. Tissue expanders have therefore cemented their place as one of the techniques in reconstructive surgery.
[1]. I Pitanguy, NF Gontijo de Amorim, HN Radwanski, JE Lintz, Repeated expansion in burn sequelBurns 2002 28:494-99. [Google Scholar]
[2]. C Radovan, Tissue expansion in soft tissue reconstructionPlast Reconstr Surg 1984 74:482-92. [Google Scholar]
[3]. ED Austad, KA Pasyk, KD McClatchy, GW Cherry, Histomorphologic evaluation of guinea pig skin and soft tissue expansionPlast Reconstr Surg 1982 70:704-10. [Google Scholar]
[4]. KA Pasyk, LC Argenta, ED Austad, Histopathology of human expanded tissueClin Plast Surg 1987 14:435-45. [Google Scholar]
[5]. KA Pasyk, ED Austad, KD McClatchey, GW Cherry, Electron microscopic evaluation of guinea pig skin and soft tissues “expanded” with a self-inflating silicone implantPlast Reconstr Surg 1982 70:37-45. [Google Scholar]
[6]. LC Argenta, MW Marks, KA Pasyk, Advances in tissue expansionClin Plast Surg 1985 12:159-71. [Google Scholar]
[7]. LA Lantieri, N Martin-Garcia, J Wechslar, M Mitrofanoff, Y Raulo, JP Baruch, Vascular endothelial growth factor expression in expanded tissue: a possible mechanism of angiogenesis in tissue expansionPlast Reconstr Surg 1998 101:392-98. [Google Scholar]
[8]. JP Pietila, REA Nordstrom, PJ Virkkunen, PEJ Voutilzinen, AE Rintala, Accelerated tissue expansion with the “overfilling technique”Plast Reconstr Surg 1988 81:204-07. [Google Scholar]
[9]. RD Goldstein, SH Schuster, Methylene blue: a simple adjunct to aid in soft tissue expansionPlast Reconstr Surg 1987 80:452 [Google Scholar]
[10]. EK Manders, MJ Schenden, JA Ferrey, OT Hetzler, TS Davis, WP Grahm, Soft tissue expansion: concepts and complicationsPlast Reconstr Surg 1984 74:493-507. [Google Scholar]