According to world report-2013 published by United Nations office on drug and crime (UNODC), about 16.5 million, or 0.4 % of world adult population (15-64 y of age), used illicit opioids in year 2011 [1]. Over the past few years consumption of opiate preparations including heroin has become a serious problem in Kashmir. In comparison to 9.5% use of opiate based preparations during 1980 in Kashmir it had increased to 73% (out of total addicts) in 2002 and is worse now [2]. Opioids are consumed mainly for their euphoric effect but tolerance to this effect occurs relatively rapidly and abusers primarily continue to use opioids to prevent withdrawal. The clinical opiate withdrawal scale (COWS) is usually used to classify the severity of opioid withdrawal based on the score generated [3].
Detoxification is first step towards opioid addiction treatment because it reduces the severity of opioid withdrawals and patients are found to be more motivated for under taking maintenance therapy [4]. Clonidine is a α2 agonist that works to minimize the noradrenergic hyperactivity and suppresses the dysphoric state seen in opioid withdrawal. Although orthostatic hypotension is a dose related side effect, clonidine does not produce tolerance or dependence like opioid medications do. Buprenorphine is a partial mu-receptor agonist and an antagonist at the kappa-receptor [5–7]. It has been found that antagnostic acvtivity of buprenorphine is responsible for its use in detoxification and maintenance in opioid addiction [8]. Buprenorphine being a partial agonist, has a ceiling effect on its agonist activity, which increases its safety profile and minimises its liability as well as possibility of overdose relative to full agonist like methadone hydrochloride [5–7]. Although most of the prior studies have reported buprenorphine to be generally more efficacious detoxification agent as compared to clonidine in opioid-dependence, but to our knowledge, no studies has been undertaken to explore the efficacy of these two drugs in the opiate detoxification in this region, where the pattern of opioid abuse is different (mostly diverted pharmaceutical products are being consumed), patients have a shorter history of opioid abuse and have lower degree of opioid dependence relative to pattern of opioid-dependence seen in western societies [9]. The present clinical trial was undertaken to compare the relative efficacy (as primary objective) and safety (as secondary objective) of sublingual buprenorphine-naloxone and clonidine in controlling the opioid withdrawal and craving for the abused substance.
Materials and Methods
After the approval from institutional review board the present study was conducted at De-addiction centre, Institute of Mental Health and Neurosciences, Government Medical Collage, Srinagar between March 2012 to August 2013. Treatment–seeking subjects in the age group of 15-50 years fulfilling American Psychiatric Association`s Diagnostic and Statistical Manual Disorders-1V (DSM-1V) criteria for opiate dependence, and agreeable to and capable of signing the written informed consent were admitted and included for this in-patient study [10]. Exclusion criteria included evidence of a serious psychiatric or medical illness, subjects reporting after 48 hours of last opiate use, those having any contraindication to the drugs to be given during the study period (buprenorphine-naloxone and clonidine) and patients using substances of abuse other than opioids [11,12]. Psychiatric interview and medical history taking was performed by resident psychiatrist at the start of admission. Clinical examination, including recording of vital signs were undertaken each day before giving the medication. Baseline blood investigations including hepatitis and HIV were carried out for all subjects at the start and end of the study. Opioid addiction screening was done by using immunoassay method by using “INSTANT VIEW MULTI-DRUG TEST KIT.” Initially 70 patients seeking treatment were evaluated, from which 16 subjects were excluded due to lack of participation criteria or unwillingness to participate and overall 54 compliant subjects were enrolled for the study. The participants were randomised into two groups using computer generated random numbers, one group received clonidine (group A) and the other group received sublingual form of buprenorphine and naloxone combination (Group B) [Table/Fig-1]. The period of 10 days was selected as study duration, reflecting the typical duration of acute phase of opioid withdrawal and also for the fact that the patients were not followed to see the effect of the two compared drugs on maintenance and relapse of opioid addiction [13]. The supportive medicines including ibuprofen, alprozolam and gabapentin were allowed in both the groups on demand.
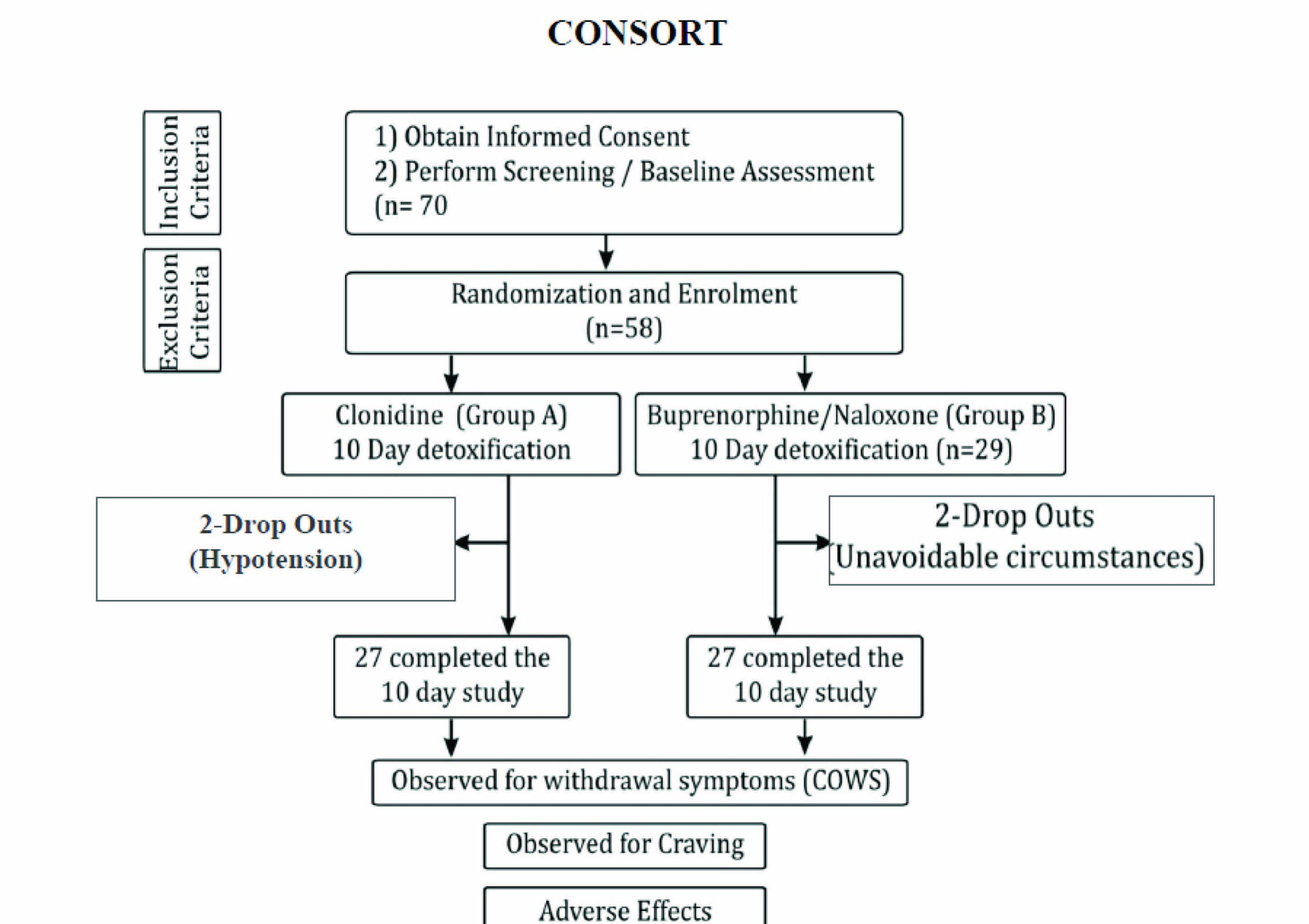
In clonidine group the subjects were given clonidine (brand name: Arkamine, containig 100 micro grams of clonidine) orally for 10 d in the dose range of 50-200 μg/day in divided doses. Starting from 50μg twice daily for day 1, the dose was increased to 50μg every six hourly from day 2 to day 4, that coincided with peak withdrawal score. Subsequently the dose of clonidine was reduced to 50 μg twice daily for day 5 to 7, and was continued as 50 μg /day from day 8 till completion of study at day 10. Blood pressure monitoring was done before giving the medication and continued for two hours every half-hourly after each dose in lying down, sitting and standing positions [14,15].
In buprenorphine-naloxone group the subjects were given combination of buprenorphine and naloxone (Bup/Nax) (brand name: Qudict, containing 2 mg of buprenorphine and 0.5 mg of naloxone) sublingually in the dose of 2.0/0.5 mg/day (1 tab.) to 8.0/2.0 mg/day (4 tab.) in two equal doses. Bup/Nax was used as 4.0/1.0 mg (2 tab.) for day 1 and the dose was escalated to a maximum of 8.0/2.0 mg/day (4 tab.) from day 2 to day 4 that coincided with peak withdrawal symptoms. The dose was reduced to 2 tab from day 5 to day 7, and was continued at the dose of 2.0/0.5 mg/day (1 tab.) from day 8 till the end of study at day 10 [16,17]. The patients were educated to place the tablet under the tongue until it had completely dissolved, which took 2-10 min [18]. Naloxone is not active when given orally and is added to buprenorphine to discourage its intra- venous use during the detoxification [19].
The primary objective of this study was to compare the efficacy of the two drugs in controlling the opioid withdrawal, for which the clinical opiate withdrawal scale (COWS) was used, which include both subjective as well as objective symptoms. The COWS was used twice on daily basis. The desire for the abused substance (craving) which was another primary objective of our study was assessed by using visual analogue scale (VAS) and the safety of the two drugs which was the secondary objective of our study was assessed by taking into consideration their side effect profile.
Statistical Analysis
The statistical analysis of data was done by Student’s t-test for the difference of means and the parametric data expressed as mean ± S.D. The nominal data was analysed by using chi-square test (X2) or Fisher’s exact test as appropriate. Any p-value of <0.05 was taken statistically significant. The analysis of data was performed by using statistical package SPSS version 20.
Results
The two groups were comparable in terms of their socio demographic parameters and the difference was statistically insignificant (p>0.05) [Table/Fig-2].
Socio-demographic variables of the study population
| Varaible | Clonidine Group | Bup-Nax Group | p-value |
|---|
| Mean Age (yrs) ±SD | 28.70±5.20 | 26.59±4.13 | 0.104 |
| Education Status (%) |
| Illiterate | 14.81 | 11.10 | |
| Middle | 29.63 | 29.63 | 0.92 |
| Under Graduate | 44.45 | 51.85 | |
| Graduate | 11.11 | 7.14 | |
| Employment Status (%) |
| Employed | 66.66 | 70.38 | 0.186 |
| Un-employed | 33.33 | 29.62 | |
| Marital Status (%) |
| Married | 29.6 | 22.2 | 0.087 |
| Un-married | 66.66 | 62.9 | |
| Divorced | 3.7 | 3.7 | |
| Y of abuse ±SD | 6.33±3.14 | 5.14±3.10 | 0.214 |
Considering the opioid withdrawal score the two groups showed statistically significant difference in their mean COWS score from day-3 onwards, with subjects in buprenorphine-naloxone group achieving lower score. From day 6 onwards buprenorphine lost its superiority over clonidine and the mean COWS score of the subjects in both the compared groups was negligible after day 6. [Table/Fig-3]. Using unpaired t-test it was observed that p-value was significant from day-3, indicating superiority of BUP-NAX in controlling the opioid withdrawals as compared to clonidine.
Comparison of average COWS scores
| Day of Detoxification | Clonidine Group Mean ± (SD) | Bup-Nax Group Mean ± SD | p-value |
|---|
| 1 | 11.37± (3.00) | 11.41± (2.71) | 0.960 |
| 2 | 22.81± (4.92) | 23.81± (4.06) | 0.420 |
| 3 | 14.85± (3.43) | 11.67± (2.40) | 0.003 |
| 4 | 9.15± (2.30) | 5.33± (1.47) | 0.001 |
| 5 | 5.811± (1.97) | 2.11± (0.80) | 0.001 |
| 6 | 2.56± (1.40) | 0.30± (0.61) | 0.001 |
The two groups were comparable with respect to the sociodemographic features as is evident with the p value(using χ2) of >0.05, which is insignificant.
Participants in BUP-NAX group were observed to achieve lower COWS scores compared to those who received clonidine during detoxification and the difference was significant, indicating the better efficacy of the former in controlling the opioid withdrawals.
Considering the efficacy of buprenorphine-naloxone and clonidine in controlling the craving for the abused substance, both the groups were comparable in terms of mean base line craving score. The two groups showed statistically significant difference with subjects in buprenorphine-naloxone group achieving lower craving scores from day 2 to day 5. The subjects had no apparent craving for the abused substance from day-5 onwards in both the compared groups and the results were statistically not comparable after day 5 [Table/Fig-4]. Using unpaired t-test significant p-value was observed from day-2, which indicates the fact that BUP-NAX was more efficient in controlling the craving for the abused substance as compared to clonidine.
Comparison of average Craving using VAS
| Day of Detoxification | Clonidine Group Mean ± (SD) | Bup-Nax Group Mean ± SD | p-value |
|---|
| 1 | 87.41± (9.84) | 90.40± (10.20) | 0.280 |
| 2 | 66.30± (10.80) | 47.40± (12.90) | 0.001 |
| 3 | 41.90± (11.80) | 22.63± (8.58) | 0.001 |
| 4 | 20.70± (10.40) | 5.60± (10.50) | 0.001 |
| 5 | 7.78± (6.41) | 1.85± (6.22) | 0.001 |
Two out of 27 patients (7.4%) in the clonidine group developed significant hypotension (B.P< 90/60 mmHg) and were taken out of the study, 22% complained of dizziness and dry mouth was reported by 11% of the subjects in this group. There is close proximity in opioid withdrawal and buprenorphine side effects and these are difficult to differentiate. 10 subjects in buprenorphine group (37%) complained of headache which was mild and tolerable, 29% reported of constipation and 22% had nausea during the course of study. Two patients in BUP/NAX group dropped out of the study because the de-addiction centre was closed when they were admitted (because of unavoidable circumstances).
Discussion
The development of effective treatment for opioid dependence is of great importance given the devastating consequences of this disorder and safe detoxification remains to be an essential initial step for it. Most of the studies comparing the efficacy of the two drugs in controlling the opioid withdrawal have found buprenorphine to be superior over clonidine. Nigam et al., [12] and Ziaaddini et al., [20] in their studies revealed the fact that buprenorphine proved to be a better drug than clonidine in controlling opioid withdrawals. In the present study it was observed that buprenorphine was superior to clonidine in controlling the opioid withdrawal during the first few days of detoxification but after day 6 the COWS score was comparable in both the groups and the subjects were withdrawal free thereafter. These findings may be due the fact that our study sample had less severe withdrawals as they were addicted to less potent opioids compared to most other studies in west were people are consuming high potency substances. The National Institute of Drug Abuse Clinical Trial Network (CTN) [21,22] compared Bup-Nax combination product to clonidine for opiate detoxification in an inpatient and outpatient treatment setting. This study showed that patients who used Bup- Nax combination drug during detoxification had lower mean COWS score (3.8 ± 2.2) compared to clonidine (7.4 ± 3.6, p<0.005). Cheskin [23] in his study reported that though there was no statistical significant difference in the COWS score between the two compared drugs but clonidine caused decrease in blood pressure and buprenorphine provided more effective early relief of withdrawal symptoms. Oreskovich [24] in his study reported that suppression of withdrawal was achieved in the first 24 hours of treatment for 50% patients on buprenorphine and in 11% of cases treated with clonidine and COWS scores were significantly less in the buprenorphine group over the day 5th of treatment. Contrary to most of the studies Collins [25] and Umbricht., [26] reported no significant difference in withdrawal severity for groups treated with buprenorphine, compared to those treated with clonidine.
Considering the effect of the two compared drugs on the craving for the abused substance, Ziaaddini et al., [20] reported that buprenorphine was found to be superior over clonidine in controlling the desire for the abused substance till 5th day of their study. Cheskin [23] found mean peak “urge” and “need” for an opioid during the first three days to be lower for those treated with buprenorphine compared to those treated with clonidine. These findings are similar to that of the present study where buprenorphine was found to be superior over clonidine in controlling the craving for the abused substance during early days of detoxification, but the subjects had no craving for the abused substance after day 5 in both the groups. According to National Institute of Drug Abuse Clinical Trial Network (CTN) [21,22], the in- patient study analysis of craving score using VAS showed statistical significant difference with Bup-Nax producing lower mean craving ratings of 29.1± 19.1 compared to clonidine where the mean craving score was 51.5 ± 28.4,p<0.005. The only study which has reported the contradictory result was the clinical trial carried out by Janiri [27], who stated that the “hunger for drug” item did not seem to be sensitive to clonidine or buprenorphine.
Significant hypotension which led to their dropout was observed in 7.4% patients in the clonidine group in our study, the other side effects observed in both the groups were mild. Oreskovich [24] did not report the exact number, but only few occasions when dose of clonidine was withheld due to hypotension. Collins [25] stated that that there were no serious adverse effects in either the clonidine or buprenorphine group.
Limitations of The Study
It was an open labelled study and blinding was not done. Since, it was an inpatient study the two drugs could not be compared in outpatient setting. The limited number of patients recruited in this study can be justified by considering the difficulty in obtaining the samples with required characteristics. The patients were not followed to see the effect of the two compared drugs on maintenance and relapse of opioid addiction. Only qualitative immunoassay test was used for the screening of opioid addiction and more accurate quantitative gas chromatography methods were not used because of lack of required resources. So we recommend further studies with more number of patients for prolonged period should be undertaken to overcome these limitations.
Conclusion
Buprenorphine was found to be more effective than clonidine in controlling the opioid withdrawal and craving for the abused substance, however it lost its superiority towards the end of the study. Considering the consumption of low potency opioids and less severe withdrawals in our patients, along with abuse potential of buprenorphine and the easy availability of clonidine, it seems that clonidine could be a good alternative to buprenorphine in our setting. Although clonidine is cost effective as compared to buprenorphine, but one has to be carefull of orthostatic hypotension which is its well known side effect.
The two groups were comparable with respect to the sociodemographic features as is evident with the p value(using χ2) of >0.05, which is insignificant.