Stress may be defined as the reaction of an organism to different stimulus. These stimuli which cause stress are called as stressors [1]. Stress is the feeling we have when under pressure, while stressors are the things we respond to in our environment. Stress would manifest itself as physiological, biochemical and behavioural changes. Over the past decades, a variety of animal models have been proposed for the study of stress and its effects. Different stressors were used in those studies using rat as a study model. One such stressor is compulsive swimming in cold water, which produced increased output of nor-epinephrine in specific parts of the brain structures and also causes alteration in blood cortisol level [2]. These stresses are linked with impairments in learning and memory that are manifested by their behaviours. They also alter brain structures involved in memory, mainly on hippocampus [3]. The other parts of the brain involved in it includes the temporal lobe, amygdala, nucleus accumbens and pre-frontal cortex [4]. It is already reported that repeated exposure to stress is always associated with eventual depletion of nor-epinephrine in hippocampus [5]. Interestingly the brain structures involved in memory are principally involved in the neurobiological response to stress. The current drug therapies for such stress induced neuronal loss are hindered due to poor efficacy and side effects. The recent re-searchers focus on the ayurvedic medicine which has proven to have no side effects. There are many herbs in ayurveda used for improving memory which includes Brahmi, Jatamanshi, Sank-hapuspi, Ashwagandha. Among these, Bacopa monniera, also referred as Brahmi, water hyssop, has been used in the Ayurvedic medicine for centuries. Habitually, it was used as a tonic to get better memory development, learning, and concentration. It is mentioned in the Ayurvedic treatise, that Brahmi is recommended for the management of mental conditions including nervousness, poor cognition and lack of attention [6]. Pharmacologically, Brahmi has combination of constituents that are valuable in mental incompetence and illnesses and are useful in the management of convulsive disorders like epilepsy. In India, Brahmi is currently recognized as effective in the treatment of mental illness and also in epilepsy [7]. Bacosides, an active ingredient in Brahmi is accountable for improving memory related functions. It is recognized with the capability to enhance the efficiency of programming the nerve impulses, thereby strengthening memory and cognition [8–10]. However, even though many studies have reported that BM enhances memory and cognition, there is diminutive knowledge on histological studies. The purpose of the present study is to evaluate the histomorphometric analysis of Bacopa monniera on cold stress induced changes in hippocampus of Wistar rats.
Materials and Methods
Animals: Twenty four male Wistar albino rats weighing 150-180 gms were divided into 4 Groups (n=6) were used. The study was conducted at BRULAC, Saveetha University, Tamil Nadu, India. The care and maintenance of the animal was as per the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA). Three rats were housed in polypropylene cage under standard laboratory conditions with food and water provided ad libitum. Before the experiment, the experimental protocol was subjected to scrutiny by an institutional Animal Ethical Committee for experimental clearance (IAEC-SU/BRULAC/RD/016/2014).
Extraction and administration of Bacopa monniera: Through proper channel, Standardized plant extract of BM was obtained from herbal manufacturer, Natural Remedies Private Limited, India. This extract was manufactured from the dried aerial parts of BM from India. The plant extract was administered 40 mg/kg orally by calculating the dosage with the body weight of the rats, using an oral feeding needle attached to a syringe. The distance from the oral cavity to the end of the xiphoid process was measured with the feeding needle, before performing the oral dosing procedure. The feeding needle glide down the esophagus with gravity alone and there was no resistance when passing the feeding needle.
Experimental design: Total 24 rats divided into four groups (n=6). Group I was control in which rats were kept under ideal laboratory conditions, Group II was given 40 mg/kg of BM extract, Group III was given cold water swim stress in which rats were forced to swim in the cold water maintained at 18±2oC till it started to sink for a period of one month and Group IV in which cold water swim stress given for a month followed by oral administration of BM extracts 40mg/kg treatment for a month. The total study was carried out for a period of 60 days. Then the animals were anaesthetized with ether and intra-cardiac perfusion of normal saline followed by 10% formal saline was done. The animals were sacrificed and their brains were dissected out from each animal. Dissection was made on ice-cold glass plate and the dissected brains were fixed in 10% chilled neutral formalin for 14 hours at 4°C. Further histological processing was done. Paraffin embedded sections of the hippocampal region with 10 μm thickness were cut on rotary microtome and stained with hematoxylin and eosin for demonstration of nerve cell bodies.
Histological studies: Quantitative analysis of neuron cell bodies in the pyramidal cell layer of CA-1 region of hippocampus was performed, using calibrated ocular micrometer. The cell count was corrected by using the Abercrombie’s formula. Darkly stained, shrunken cells and cells with fragmented nuclei were excluded from counting. Round, medium or large and clear cells with distinct nucleus were counted. Only adequately impregnated CA-1 pyramidal neurons were selected for study. The first six neurons in each animal when moving from medial to lateral in the sections were selected for quantitative analysis. Camera Lucida drawings of dendritic arborization were made at a magnification of 400x. According to a method, concentric circles were superimposed over the drawings of dendritic tree with increasing radius equal to geometric equivalent of 20 micron at a magnification of 400X.The number of dendrites intersecting each circle was counted and com-pared group wise [11].
Histomorphometry Studies
Diameter of Cells: The diameter of the cells of the CA-1 of the hippocampus was calculated. The diameter of the cell was calculated using the formula: Diameter of a cell = Axial ratio × Calibration constant, where, Axial ratio equals maximum length + maximum breadth/2. The maximum length and maximum breadth of cells was calculated using ocular micrometer. Then, the diameter of the cells of CA- 1 region of hippocampus was calculated for all animals (n=6) in a group and the results were tabulated.
Total number of cells in the square: The stained sections of CA-1 region of the hippocampus were focused under 40x objective lens of a microscope. As the calibration constant varies with different microscope and objective, one microscope with the same objective and magnification was used. Counting of neurons was done under 400X magnifications. The pyramidal cell of CA-1, which is a continuation of Cornu Ammonis region-3, was counted. The cell region for calculation was selected using random selection technique from the serial sections made for each group.
Packing density of cells: The packing density was calculated by the following formula ND = NA/ A (d+t) Where ND – numerical density or number of cells per cubic mm, NA - Average number of cells per sq mm. A - Area of reticule in sq mm, t - Thickness of the section in mm, d - Mean diameter of the cells in mm.
Statistical Analysis
Values are expressed as Mean ± SEM (n = 6). One-way analysis of variance followed by Student-Newman-Keul’s multiple comparisons test was used for the comparison of means. A probability of 0.05 and less was taken as statistically significant. In the graph same alphabetical characters are mutually significant. The analysis and plotting of graphs were carried out using Sigma Plot 12 (Systat Software Inc., USA).
Result
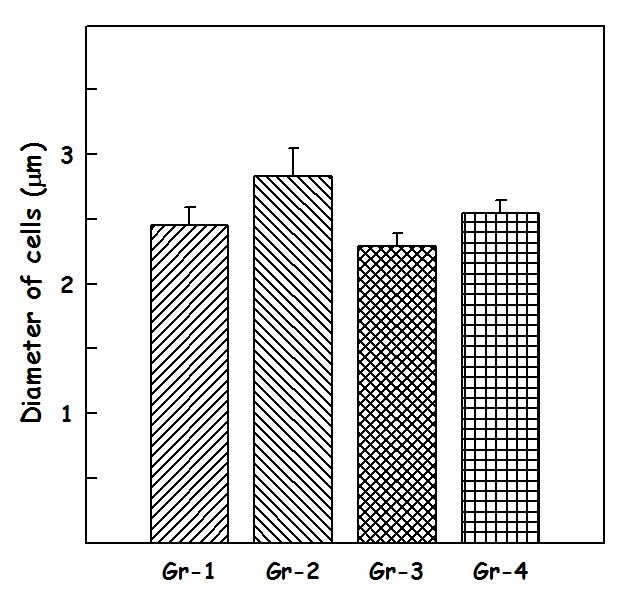
Results of diameter of cells (d) in CA – 1 region: The diameter of cells [Table/Fig-1] in CA 1 region, in group I was 2.45±0.14microns, in Group II was 2.83±0.21 microns, in group III was 2.29±0.10 microns and in group IV was 2.54±0.10microns. One-way-ANOVA test was performed for diameter of cells in CA – 1 region between the groups showed that there is not a statistically significant difference between groups (p=0.091).
The values of Diameter of cells are in units of microns
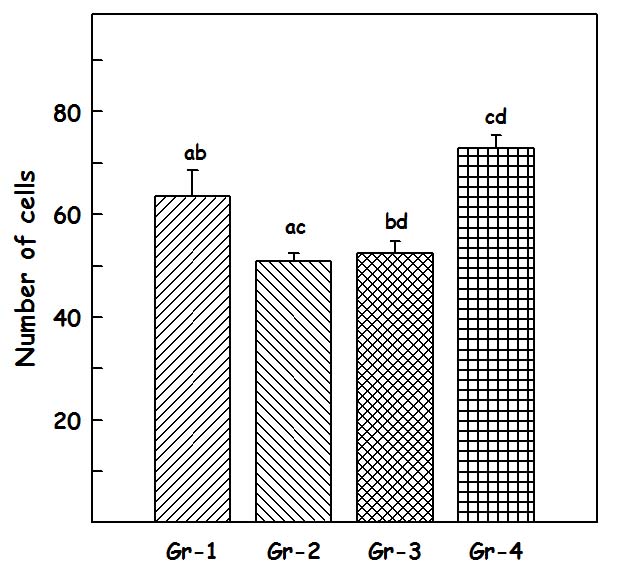
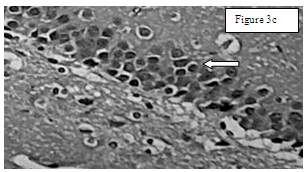
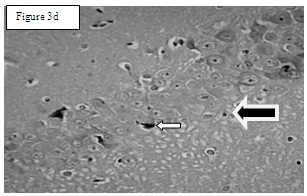
Results of total number of cells in CA – 1 region: The total number of cells [Table/Fig-2] in CA 1 region in Group I was 63.33±5.7194 [Table/Fig-3a], in group II 81.83±5.23 [Table/Fig-3b], in Group III 50.83±1.579 [Table/Fig-3c] and in Group IV 65.5±5.43 [Table/Fig-3d]. One-way-ANOVA test showed that there is statistically significant difference (p= 0.002). Student–Newman–Keuls method was used for multiple comparisons which showed that Group II are statistically significant when compared to other groups (p<0.05).
The values of number of cells represent average number of cells present per 0.14sq mm, which is the area covered by the reticule
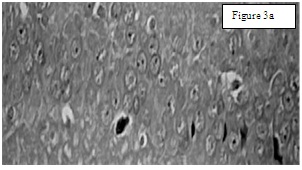
Group 1. Neuronal cells with centrally placed nucleus
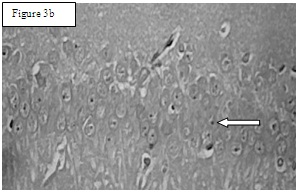
Group 2. Arrow indicates normal cells with centrally placed nucleus and also increased number of cells
Group 3. Arrow indicates apoptotic cells (karyopyknosis) that are darkly stained
Group 4: Short Arrow indicates apoptotic cells and long arrow (black color shaded) indicates normal cells
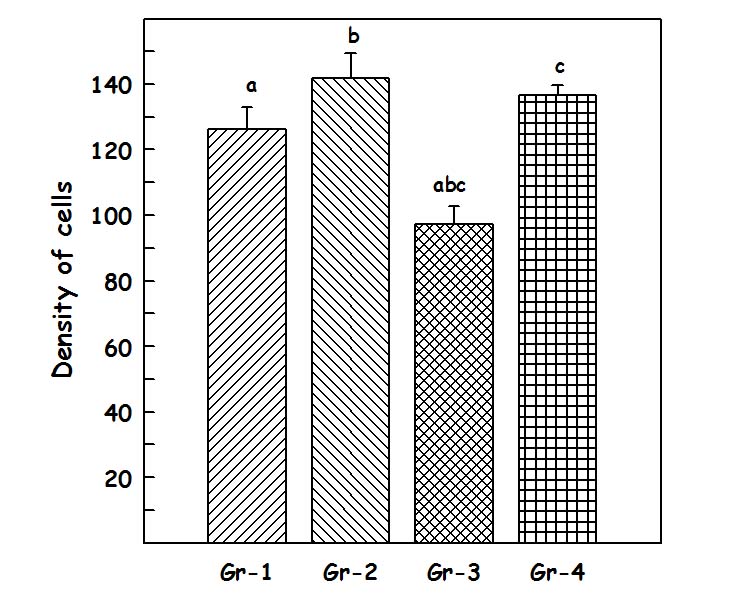
Results of packing density of cells in CA – 1 region: The packed density [Table/Fig-4] of CA-1 region in Group I was126.33±6.73, in Group II 141.83±7.51, in Group III 97.16±5.5463 and in group IV 136.66±2.89. One-way-ANOVA test showed that there is statistically significant difference (p= <0.001). Student–Newman–Keuls method was used for multiple comparisons which showed that Group II and Group IV are statistically significant (p<0.05) when compared to Group III.
The values of packed density in units of (×103/ cubic mm)
Discussion
Hippocampus is one of the most important component of the brain of humans and other mammals. The parts of the brain involved in stress include hippocampus, the temporal lobe, amygdala, nucleus accumbens and pre-frontal cortex [4]. These stresses are linked with impairments in learning and memory that are manifested by their behaviours. Interestingly the brain structures involved in memory are principally involved in the neurobiological response to stress [10]. repeated exposure to such stress is associated with an eventual depletion of nor-epinephrine in the hypothalamus and hippocampus [5]. Researchers had conducted numerous studies with medications to reduce the impact of stress. To the surprise most of them identified natural compounds that serve as nootropic agents. To date, pharmaceutical companies have been investing enormous resources in the identification of agents that could possibly improve debilitating disorders and slow the onset of mental retardation. Bacopa monniera is one such plant with wide medicinal properties that is being used as a treatment for memory-related disorders [6]. Several researchers have carried out systematic chemical examinations of this plant to prove its importance in the brain related lesions. Detailed investigation first reported the isolation of the alkaloid ‘brahmine’ from Brahmi [12] Later, several compounds have been isolated together with nicotine, herpestine, betulic acid, stigmastarol, beta-sitosterol, as well as bacosides and bacopasaponins [6,12]. Extensive investigation on bacosides, especially bacosides A and B was studied [7,13]. Dosage of 40mg/kg was given in our study as studied to be safe and potentially improves the memory [13]. Bacopa monniera is used in medicine for the treatment of various nervous system ailments such as insomnia, anxiety, epilepsy, and hysteria. Preclinical and many clinical studies have shown that BM improves recollection and mental function [14]. In the present study cold water swim stress, was given to rats which causes major neuronal loss in CA-1 region than CA3 region [15]. Histomorphometric study reveals an increase in the density of neuronal cells in CA-1 region. This ability for connectivity was by participation of dendritic spine in the formation of new associative memories [16]. In this study, total number of neuronal cells and the density in group II were more when compared to the other group’s. Earlier reported a transient increase in the spine density in the dentate gyrus improves spatial learning [17]. Present study shows that total number of the cells in Group II is statistically significant when compared with the others groups, which means that when BM added as adjuvant to our daily diet can possibly improve memory. Group IV is significant to Group I and Group III which clearly indicates BM prevents the neuronal cell death in the hippoccampal region caused by stress, producing neuroprotective effect thereby improving the memory. These hippocampal neurons are functionally included with existing neuroanatomical path has been reported [18], and are positively correlated with hippocampus. Dendritic spines are known as potential source of improving contact between excitatory neurons thereby improving cognition [19–21].
Conclusion
In our present study, the herb BM is an efficient drug in producing neuroprotective effects in stress induced hippocampus of Wistar albino rats. As stress affects most of the neurons in cornu ammonis region of hippocampus, the herb BM may be used as a supplement to alleviate the harmful effects, thereby improving memory. Hence, the herb BM can possibly be used as an adjuvant to improve memory to combat stress in our day to day life.