A positive correlation between the biofilm formation and drug resistance in the form of extended spectrum β - lactamase (ESBL) bla PER-1 gene among the A. baumannii isolates has already been reported [5]. Therefore, the present study was undertaken to study biofilm formation and correlation between biofilm formation and the multiple drug resistance in A. baumannii.
Materials and Methods
Bacterial isolates and antimicrobial susceptibility testing
The present study was conducted in Department of Microbiology, Konaseema Institute of Medical Sciences (KIMS), Amalapuram, for seven months duration period from January 2014 to July 2014. A total of 72 isolates of A. baumannii were obtained for study from various clinical specimens like endotracheal aspirates, cerebrospinal fluid, pus, wound swabs, urine, blood culture specimens, body fluids etc. from the patients admitted in hospital. All the isolates were processed and confirmed by conventional microbiological methods using phenotypic test [6–8]. All 72 isolates were tested for antimicrobial susceptibility with amikacin (30 μg), ampicillin-sulbactam (10/10 μg), ceftazidime (30 μg), ciprofloxacin (5 μg), imipenem (10 μg), piperacillin (100 μg) by Kirby Bauer disc diffusion method. Interpretation of antimicrobial susceptibility testing by disc diffusion test was done as per Clinical Laboratory and Standard Institute (CLSI) Guidelines [9,10].
Biofilm formation [
11,
12]
This was determined by microtitre plate method. Each isolate was grown overnight in trypticase soy broth (TSB) with 0.25% glucose at 37oC. The overnight growth was diluted in a ratio of 1:40 in TSB-0.25 % glucose. Two hundred microlitre of cell suspension was inoculated in sterile 96 well polystyrene microtitre plates. After 24 h of incubation, the wells were gently washed three times with 200 microlitre of phosphate buffered saline (PBS) then dried in an inverted position and stained with 1% crystal violet for 15 min. The wells were rinsed again in 200 microlitre of ethanol-acetone (80:20 v/v) to solubilise crystal violet. The optical density at 620 nm (OD 620) was determined using microplate reader. Each assay was performed in triplicate and the average optical density was considered.
The following values were assigned for biofilm determination:
Non-biofilm producer: OD620 < 0.275
Weak biofilm producer: 0.275 ≤ OD 620<0.55
Medium biofilm producer: 0.55≤ OD620<0.825
Strong biofilm producer: 0.825 ≤OD620
The number 0.275 was chosen for guideline because it was three standard deviations above the mean OD (0.303) of a clean microtitre plate stained by the above method.
Results
In the present study, a total of 45 (62.5%) isolates produced biofilm. The results of quantitative assay for biofilm formation are shown in [Table/Fig-1]. Drug resistance pattern shows isolates are least resistant to ampicillin-sulbactam (25%) and highest resistant for piperacillin (84.7%). Details of resistance pattern for other antibiotics are mentioned in [Table/Fig-2].
Biofilm formation in A. baumannii
| Biofilm formation | No. of isolates (%) |
|---|
| 1 | Formers | 45 (62.5) |
| - Weak | 03 (6.67%) |
| - Medium | 05 (11.11%) |
| - Strong | 37 (82.22%) |
| 2 | Non formers | 27 (37.5) |
Resistance pattern to antimicrobials in A.baumannii
| Name of antimicrobial | No of resistant Isolates (%)n=72 | Biofilm positive resistant isolates (%) | Biofilm positive resistant isolates (%) |
|---|
| 1 | Ampicillin Sulbactam | 18 (25) | 10 (55.5) | 8 (44.5) |
| 2. | Piperacillin | 61 (84.7) | 26 (42.6) | 35 (57.4) |
| 3. | Amikacin | 58 (80.5) | 31 (53.4) | 27 (46.6) |
| 4. | Ciprofloxacin | 52 (72.2) | 26 (50) | 26 (50) |
| 5. | Ceftazidime | 48 (66.6) | 25 (52.08) | 23 (47.92) |
| 6. | Imipenem | 26 (36.1) | 10 (38.4) | 16 (61.6) |
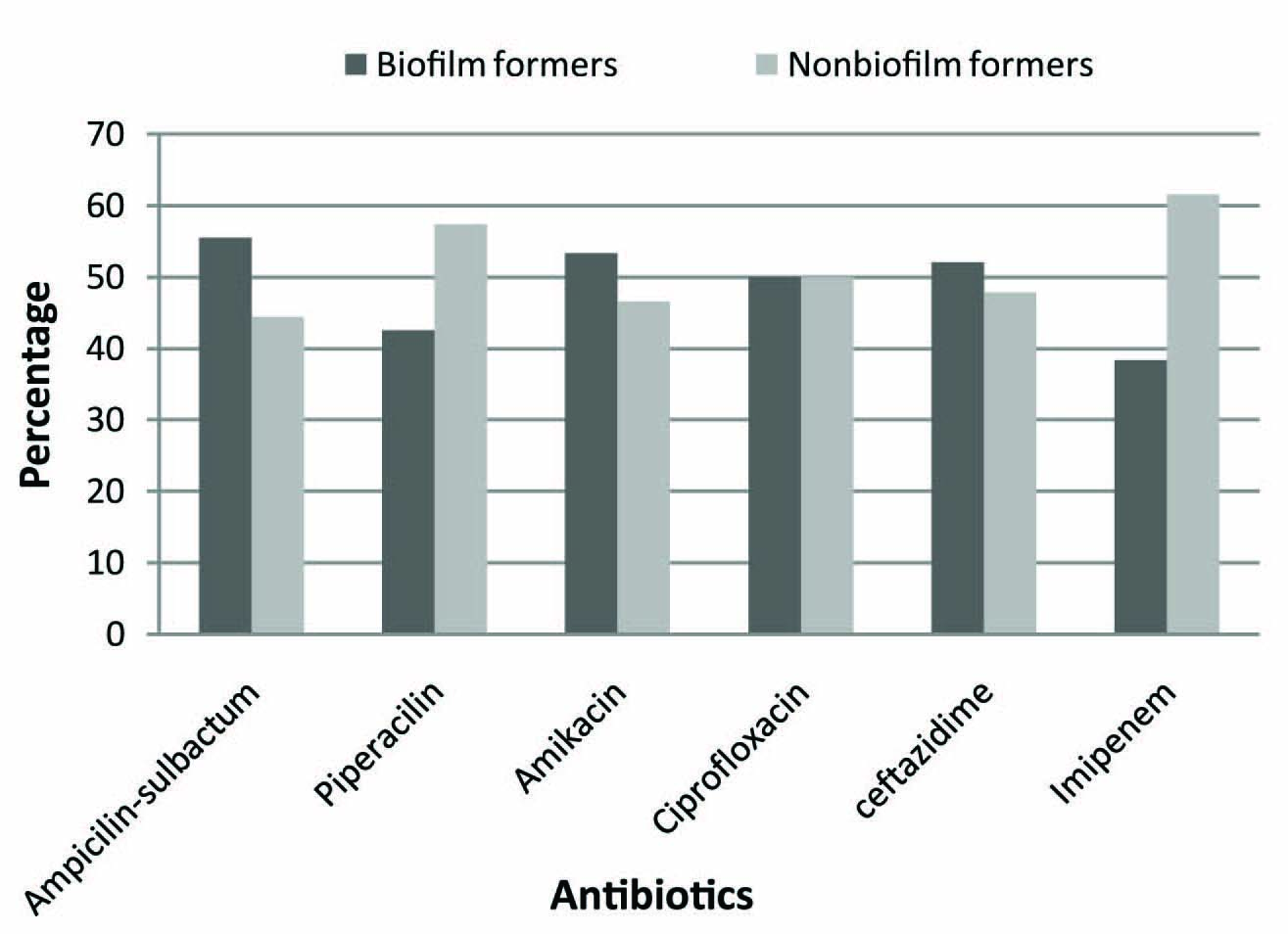
Comparison of resistance pattern of biofilm forming A. baumannii isolates with non biofilm formers is shown in [Table/Fig-3]. Biofilm formers showed greater resistance for ampicillin- sulbactam, amikacin, ciprofloxacin and ceftazidime as compared to imipenem and piperacillin. In all 65 (90.3%) isolates showed multiple drug resistance [Table/Fig-4]. Further correlation between multidrug resistance and biofilm formation was analysed statistically by Chi–Square Test and p-value (0.0004) was found to be significant [Table/Fig-5].
Distribution of A.baumannii resistant isolates: Biofilm vs. non biofilm formers
Frequency of multiple drug resistance in A. baumannii
| No .of resistant antimicrobial agents | No. of isolates (%) n=72 | Biofilm positive resistant isolates |
|---|
| 0 | 1 (1.4) | 1 |
| 1 | 6 (8.3) | 4 |
| 2* | 8 (11.1) | 4 |
| 3* | 13 (18.1) | 9 |
| 4* | 20 (27.8) | 16 |
| 5* | 19 (26.4) | 9 |
| 6* | 5 (6.9) | 2 |
| Total | 72 | 45 |
* No. of MDR (resistant to two or more antimicrobials) isolates is 65 (90.3%)
Correlation between multiple drug resistance and biofilm formation in A.baumannii (statistical analysis)
| Biofilm formation | Multiple drug resistance |
|---|
| Positive | Negative | Total |
|---|
| Positive | 40 | 5 | 45 |
| Negative | 25 | 2 | 27 |
| Total | 65 | 7 | 72 |
(p-value = 0.0004; p-value < 0.05 is significant by Chi – Square Test)
Discussion
Of the “newer” pathogens now recognized, Acinetobacter baumannii plays a significant role in colonization and infection of patients admitted to hospitals. They have been implicated in a variety of nosocomial infections, including bacteremia, urinary tract infections and secondary meningitis but their predominant role is as agents of nosocomial pneumonia, particularly ventilator associated pneumonia in patients confined to intensive care units (ICU’s). Such infections are often extremely difficult to treat for the clinician because of widespread resistance of the virulent organism to a large number of antibiotics [13].
A.baumannii clinical isolates have the ability to survive long stretches of time under highly desiccated conditions on abiotic surfaces [14,15]. Rodriguez et al., showed that the ability to form biofilms on abiotic surfaces is a common trait among A. baumannii strains, particularly isolated from catheter-related urinary tract infection or bloodstream infection as well as a case of shunt-related meningitis [4]. Biofilm is a group of microorganisms in which bacterial cells are adherent to each other and embedded within a self-produced matrix of extracellular polymeric substance or exopolysaccharide (EPS) [16].
Generally, two properties are often associated with biofilm producing bacteria, namely, the increased synthesis of exopolysaccharide and the development of antibiotic resistance [16]. Increased production of EPS in A. baumannii is likely to create a protective environment causing difficulty in antibiotic penetration leading to development of resistance. Also, there are differences in the cellular physiology of cells within the biofilm that results in increased drug resistance [17]. The ability of bacterial cells to transfer genes horizontally is enhanced within biofilm communities, thereby facilitating the spread of antibiotic resistance [18].
We selected to study biofilm formation by clinical isolates of A. baumannii and its correlation with multiple drug resistance in order to understand the ability of A.baumannii to persist in the hospital environment to cause outbreaks. In the present study, 62.5% isolates produced biofilm as assessed by microtitre plate method. Similar occurrence of 63% and 62% biofilm formers have also been reported by Rodriguez et al., [4] and Rao et al., [19] respectively. A recent study, although done by tube method reported positivity of 50% for biofilm formation in A.baumannii isolates [20].
In the present study, biofilm formers showed greater resistance to ampicillin- sulbactam, amikacin, ciprofloxacin and ceftazidime as compared to imipenem and piperacillin. Nahar et al., has reported even 100% resistance to amoxicillin, ceftriaxone, ceftazidime, cefuroxime, and aztreonam in biofilm forming Acinetobacter species. Also in the same study, resistance to gentamicin, amikacin, netilmicin, ciprofloxacin and imipenem was higher among biofilm forming Acinetobacter isolates particularly from ICU patients [21]. In the present study, less resistance to imipenem and piperacillin in biofilm formers could be due to ability of both the antibiotics to penetrate the biofilm, inhibiting the bacterial growth. This finding is supported by a research on permeation of antibiotics through biofilms which demonstrates that piperacillin and imipenem have relatively high permeation with penetration through biofilm as compared to aminoglycosides or fluroquinolones [22].
Considering multiple drug resistance as resistance to two or more antimicrobial agents [23], 90.3% isolates showed multiple drug resistance in the present study. Statistical analysis proved a significant correlation between multiple drug resistance and biofilm formation, which is in accordance to the findings reported by various studies [19–21]. Conversely, a reciprocal study to investigate various virulence factors in known MDR (ciprofloxacin-imipenem-trimethoprim/sulfamethoxazole-resistant) A.baumannii isolates, reported that 24.6% MDR isolates produced biofilm thereby suggesting a synergistic relationship of biofilm formation with multiple drug resistance [24].
Thus, A. baumannii strains capable of forming biofilm might be selected under antibiotic pressure, or conversely, A. baumannii might acquire resistance to multiple drugs within biofilm communities. In either event, the high colonizing capacity of A. baumannii, combined with its resistance to multiple drugs, will contribute to the organism’s survival and further dissemination in the hospital setting. Thus, the functions of the biofilm formed by A. baumannii encompass its ability to resist antimicrobial therapies as well as to protect from external stresses such as dehydration and limited nutrient availability [17,18].
Rao et al., reported a significant association between multidrug resistance and biofilm, although the study shows that the presence of blaPER-1 is more critical for cell adhesion than the formation of bacterial biofilms on abiotic surfaces [19]. It was observed that cell adhesiveness and biofilm formation on plastic is higher in strains harboring the blaPER-1 gene than in those that do not harbor this genetic trait. Further, the level of expression of this gene, as determined by reverse transcription-PCR, is positively correlated with the level of biofilm formed on plastic and the adhesiveness of bacteria to human epithelial cells [25].
Conclusion
This study concludes that there is a positive correlation between biofilm formation and multiple drug resistance in A. baumannii. This study needs further molecular support like detection of blaPER -1 gene to provide a quantum leap in our understanding of not only biofilm formation but also its virulence, responsible for multidrug resistance and survival in hospital environment.
* No. of MDR (resistant to two or more antimicrobials) isolates is 65 (90.3%)
(p-value = 0.0004; p-value < 0.05 is significant by Chi – Square Test)