Colorectal cancer (CRC) is the third most commonly diagnosed cancer in the world. Globally more than 1 million people suffer from colorectal cancer annually resulting in about 0.5 million deaths, and making it the second most common cause of death due to cancer [1]. The role of estrogen in CRC is being researched with great interest especially in relation to the patho-physiology of cancers of breast, prostate, endometrium as well as colon and rectum [2]. The exact mechanism of action of estrogen in CRC remains a debatable issue [3]. According to some investigators, endogenous estradiol plays an important role in the biological pathway of CRC, and circulating levels of estradiol are associated with an increased risk of cancer [4]. Estrogen impairs the mucosal response to inflammatory damage in colitis and thus promotes inflammation-associated cancer development [5]. The expression of estrogen receptors (ER) (alfa and beta) are being explored in this regard. Estrogen receptor (ER) alfa act as a modulator of cellular energy metabolism and controls osteopontin promoter expression in human CRC [1]. On the other hand ER beta promotes colonic growth, and therefore beta antagonists (raloxifen) are being thought of in decreasing the rate of colonic carcinogenesis [6]. Oral contraceptive use significantly decreases the rate of ER-beta positive CRC tumour, but similar action is not seen in ER- beta negative cases [7]. Aromatase are expressed in colon carcinoma tissues and thus tumour cells are acting as source of estradiol production in CRC patients [8]. Alteration of estrogen induced signal transduction pathway has preventive and therapeutic effect for obesity-associated colon cancer [9]. Researchers also found that there is a significant correlation between serum estradiol level and mismatch repair gene activity in colon cancer tissue [10]. In cancer cells, fatty acid synthase act as a metabolic oncogene and therefore it causes growth to the tumour cells. Hormones such as estrogen contribute to the transcriptional regulation of fatty acid synthase expression [11].
Our objective was to compare the serum estradiol levels in diagnosed male patients of colorectal cancer, with age-matched controls; and to study the estradiol levels across the different stages of CRC to evaluate whether stage-wise disease progression may be related to estradiol levels, and if estradiol can possibly be considered as a biomarker in CRC.
Materials and Methods
A cross-sectional study was conducted from January, 2012 to March, 2013 at the Department of Biochemistry, at a tertiary care hospital in northern India. Ethical clearance was obtained from the Institutional Ethical Committee. Informed written consent was obtained from each participant.
Fifty one (n=51) preoperative male patients with CRC who attended the clinics of the Radiotherapy and Surgery departments during the study period for treatment were included in the study. All cases were in the pre-treatment stage. Fifty (n=50) age matched healthy males, from among persons accompanying the patients were also enrolled as the comparison group.
About 95% of the CRC patients attending the clinics in the Departments of Radiotherapy and Surgery (recruitment sites) are males. The decision of excluding female patients was also due to the fact that serum estradiol levels physiologically vary in females, depending on age and menstrual cycle; thus a matched control group would be extremely challenging to achieve.
Diagnosis of CRC was confirmed by biopsy and surgical staging (TNM staging: T- Tumour, N- Node, M- Metastasis; I, II, III and IV) for each case. Patients diagnosed with other benign colorectal conditions like ulcerative colitis, Crohn’s disease or other diseases like prostate cancer as well as the patients who were on drugs like estrogen, progesterone, flutamide, testosterone were excluded from the study. Venous blood samples were taken from each case and control in between 7am to 9am in the morning. Centrifugation at 3000 rpm for 10 min was done. Serum samples were kept frozen at – 20°C until day of the assay.
Serum estradiol level was measured by direct Chemiimmun-ofluroscence technique by using ADVIA Centaur estradiol assay, a competitive immunoaasay (kit supplied by SIEMEN and normal range for males = 11.6 - 41.2 pg/ml).
Surgical staging was based on the classical TNM classification, determined by initial tumour spread (T), lymph nodes involvement (N) and, presence of metastasis (M). Superficial lesions that did not involve the regional lymph nodes and did not penetrate the submucosa (T1) or the muscularis (T2) were designated as stage I (T1–2N0M0) disease; tumours that penetrated through the muscularis but did not spread to lymph nodes were categorized as stage II disease (T3N0M0); regional lymph node involvement defined stage III (TXN1M0) disease; and metastatic spread to distant sites such as liver, lung, or bone indicated stage IV (TXNXM1) disease [12].
Out of the 51 patients, 28 were colon cancer cases, while 23 suffered from rectal cancer. Number of patients in each stage (TNM) were n=10 (stage I), n=17(stage II), n=13(stage III), and n=11(stage IV). We also stratified the classes according to age (<30 y, 30-45, 45-60 and >60 y). Level of estradiol was measured in each sub groups and assessed for any significant differences between the groups.
Sample size
The sample size estimation was based on a previous (unpublished) study by the investigators. A sample size of 46 in each group had 95% confidence level and 80% power to detect a 2-sided significant mean estrogen level difference of 12.6 pg/ml between the cases (sd=24.1) and controls (sd=18.9).
Statistical Analysis
The data were analysed and represented as mean (sd); students t-test and the analysis of variance (ANOVA) were applied as the test of significance (2-tailed); p < .05 was considered significant. In cases where ANOVA was significant, the post-hoc Bonferroni test was done. All analyses were performed using SPSS version 17.0 (SPSS, Inc., Chicago, Illinois).
Result
The mean age of the patients (cases) was 49.8 years (sd=15.3) years, and that in the comparison group was 47.2 years (sd=13.9). The mean serum estradiol level among CRC patients was 43.4 pg/ml, (sd=27.1); and it was significantly higher (p<0.0001) compared to the controls (mean=24.7 pg/ml, sd=17.5).
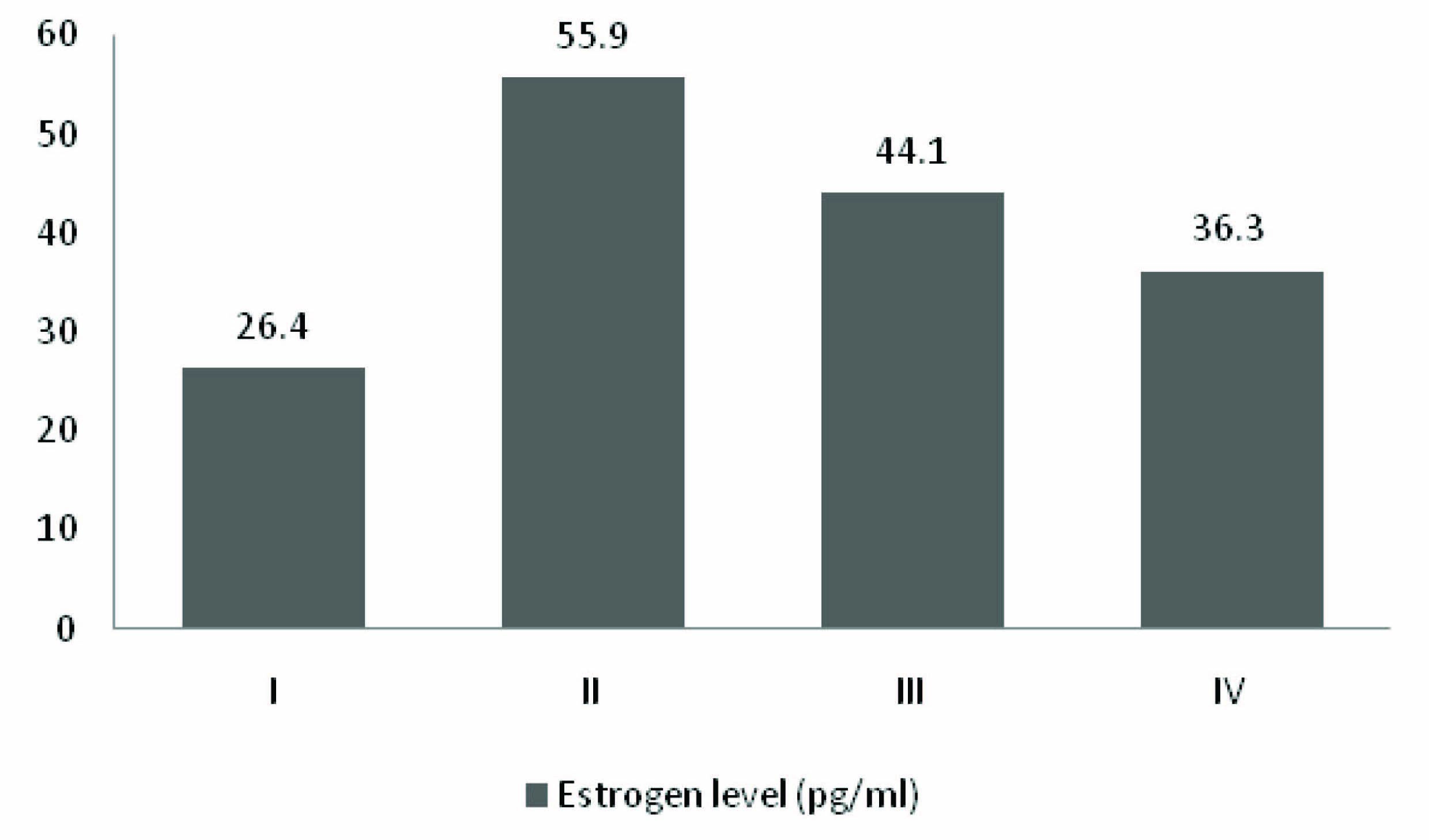
[Table/Fig-1] demonstrates that there were no significant differences in age and estradiol levels between the two types of cancer (colon & rectal) among the cases (n=51). [Table/Fig-2] describes the age and estrogen levels across the four TNM stages of colorectal cancer (n=51). The mean estradiol level was highest in Stage II (mean=55.9 pg/ml, sd=15.5); followed by Stages III (mean=44.1 pg/ml, sd=24.9), IV (mean=36.3 pg/ml, sd=30.0) and I (mean=26.4 pg/ml, sd=38.8) [Table/Fig-3]. However, significant difference was obtained only between Stages I and II.
Contrasting age and estrogen levels across types of CA (colon & rectal) (n=51)
| Parameters | Type of CA | p-value (independent samples t test) |
|---|
| CA Rectum (n=23) | CA Colon (n=28) |
|---|
| Age (years) | 47.1 (16.3) | 52.04 (14.2) | 0.25 |
| Estrogen level (pg/ml) | 40.19 (31.50) | 38.78 (23.33) | 0.86 |
Contrasting age and estrogen levels across different stages of colorectal carcinoma (n=51)
| Parameters | Stages of colorectal cancer (n=51) | p-value (ANNOVA) |
|---|
| Stage I (n=10) Mean(SD) | Stage II (n=17) Mean(SD) | Stage III (n=13) Mean(SD) | Stage IV (n=11) Mean(SD) |
|---|
| Age (years) | 48.8 (16.3) | 54.1 (13.6) | 44.9 (15.1) | 49.9 (17.0) | 0.45 |
| Estrogen level (pg/ml) | 26.4 (38.8) | 55.9 (15.5) | 44.1 (24.9) | 36.3 (30.0) | 0.048** |
**post-hoc Bonferroni test: Stage I v/s Stage II = p<0.05
Levels of serum estradiol across the four TNM stag
Discussion
It is known that pre or post-menopausal women under hormone replacement therapy are at a lower risk of developing colorectal cancer [3]. But the question remains on the role (if any) of estrogen, and the underlying mechanism, in development of CRC. Our study found that estradiol levels were raised among CRC patients compared to controls. Gunter MJ et al., had stated two important biological pathway of colorectal cancer. Involvement of endogenous estradiol was one of them [4].
According to English MA et al., during estrogen metabolism active estradiol (E2) is converted to less active estrone (E1) by the enzyme 17 beta hydroxyl steroid dehydrogenase (17 beta-HSD). When the enzyme activity is less, estrogen remains in its active form, estradiol leading to cell proliferation of colon and rectal cell growth. They also found that when a less active form of estrogen, estrone is administered, cellular proliferation in colon and rectum is arrested [13,14].
Di Domenico M et al., has reported that estradiol was the main activation factor among other steroids in colonic carcinoma derived Caco-2 Cell line. According to them it induced signal transduction through c-src, c-yes/MAP kinase (mitogen activated protein kinase) pathway by activating c-src related tyrosine kinase and serine/threonine kinases ultimately leading to cell growth [15]. An animal study reported that by estradiol functions by increasing the level of ornithine decarboxylase (ODC) mRNA and c-myc (protooncogene) mRNA, and promotes colorectal growth [16]. Sato R et al., concluded that colon carcinoma cells expresses immunoreactivity to aromatase enzyme which is an important enzyme for estrogen production [8]. According to another animal study, estradiol treated mice showed increased numbers of polyps in colon. Researchers stated that estradiol promoted tumour progression in those mice leading to invasive colonic adenocarcinoma formation [5]. Huanlei Wu et al., suggested that estrogen individualy acts as a risk factor for CRC in male patients [17].
Adenoma as well as carcinoma derived cell line of colon expresses estrogen receptor [7,18]. Some researchers believe that there might be possible tropic action of estrogen towards premalignant colonic epithelium, and estrogen exposure gradually converted it to carcinomatous growth [18]. Mechanism of action of estrogen are mediated by two receptors- ER-alfa and beta, through signal transduction and by regulating the transcription rate at genetic level. Some studies have stated that colon adenocarcinoma expressed higher level of mRNA as well as protein- ‘ER- beta’ in comparison to normal colonic mucosa. Therefore selective estrogen receptor modulator (SREM) raloxifene could be used to decrease the growth of CRC [6].
A handful of contradicting findings have also been reported in published literature. According to Boujadi S et al., Estrogen related receptor-alfa controls the expression of osteopontin which is a component of extra cellular matrix in colorectal tumour. Hence there might be a possibility that ERR-alfa is also associated with carcinogenesis; and use of relevant antagonist have been suggested [1]. Some studies have also stated that it is not estradiol but estrone which is related to increased risk of colorectal cancer [19]. Foster PA postulated an initial cancer protective role of estrogen [3].
Our study found increased levels of estradiol in CRC patients with significant difference between stage II (raised level) and stage I. The exact underlying mechanism remains poorly understood, given the available literature. It is possible that the increased levels of serum estradiol found in our CRC patient population may serve as a diagnostic marker.
Our study suffers from limited sample size and inclusion of male sex only, the justification for which has been provided earlier. Though, inclusion of female patients (both pre and post menopausal) might provide further interesting results. Notwithstanding few limitations, the current study demonstrates raised levels of serum estradiol among Indian male CRC patients, providing a basis of future research to assess prognostic value of estradiol, and in prediction of tumour recurrence.
Conclusion
Estradiol in CRC is a well researched topic in recent era. In view of the inconsistency associated with other tumour markers in CRC, estradiol could be considered as a possible diagnostic marker. Furthermore, anti estradiol drug therapy could be explored as a treatment option in CRC patients. Our study bears some indication that therapeutic use of estradiol receptor antagonists in the treatment of CRC may be a subject of future research.
**post-hoc Bonferroni test: Stage I v/s Stage II = p<0.05