Spin echo T1 and Magnetization transfer T1 sequences (MTT1) with saturation pulse are routinely used after intravenous gadolinium contrast injection to evaluate various intracranial pathologies including leptomeningitis. The T1 shortening effect (relaxivity) leads to contrast enhancement [1–3]. However, in the last decade, post-contrast Fluid Attenuated Inversion Recovery (PCFLAIR) has emerged as a useful sequence to evaluate leptomeningeal pathologies [4–16]. The nullification of CSF signal, inconspicuous vascular enhancement as compared to T1-weighted imaging (T1WI) and some degree of T1 relaxivity effect makes meningeal enhancement easily discernible on PCFLAIR images [7,11–14]. Despite its utility in leptomeningeal diseases, there have been controversies regarding the routine use of PCFLAIR in MR practice and its relative advantage over post contrast T1WI (PCT1W) with or without fat suppression (FS) [15–18]. The aim of this study was to qualitatively and quantitatively differentiate leptomeningeal and vascular enhancement on PCFLAIR (PCFLAIR) and post contrast T1-weighted (PCT1W) with fat suppression (FS) sequences in cases of meningitis to aid in early detection of infectious meningitis.
Materials and Methods
The prospective study was approved by the research and ethical committee of the institution. The study was conducted between 2011-2012. The study group comprised of 31 patients who were diagnosed with infectious meningitis. CSF cultures were positive in clinically proven cases of meningitis. The patients who had relevant clinical history, typical CSF cytological findings/biochemical markers (Adenosine deaminase activity, D-lactate) or showed therapeutic response were strongly suspected for meningitis. Out of 31 patients, 15 cases were of tuberculous meningitis, 6 were of pyogenic meningitis and 10 of viral meningitis. The routine consent for contrast examination was taken either from the patient or the guardian (in patients who were unable to give the informed consent themselves and in children). Patients with subarachnoid hemorrhage, stroke and those who had received supplemental oxygen, gadolinium or iodinated contrast injection in the previous week were excluded from the study. The MR examination was performed on 1.5 Tesla MR System (Magnetom, Siemens, Erlangen, Germany). The imaging parameters for pre and post contrast T1-weighted (PCT1W) sequence with fat suppression (FS) were (TR-500-600ms, TE-10ms, Echo train length-58, turbo factor-2, slice thickness-5mm, interslice gap-1.5 mm, FOV-230mm, and matrix-256x173). Total imaging time was 1 min and 44 sec. Imaging parameters in pre and post contrast FLAIR sequences were- (TR-9000ms, TE eff-88ms, Echo train length-7, Turbo factor-16, TI-2500 ms, slice thickness-5mm, interslice gap-1.5 mm, FOV-230mm, phase resolution-80, phase oversampling-30, averages-1, signal to noise ratio-1, and matrix-256x173). Total imaging time was 2 min and 26 sec.
Post-contrast images were obtained after administration of intravenous gadodiamide (Omniscan, GE Healthcare) in the dose of 0.1mmol/kg body weight. PCFLAIR was acquired after PCT1W with FS sequence with a delay of nearly 3-4 min.
Images were evaluated both qualitatively and quantitatively. Qualitative evaluation was performed by the assessment of the images independently by two radiologists (AA, RA) who were blinded to the clinical history and cytological results for the presence, absence or equivocal status of meningeal enhancement. On PCT1W, the meningeal enhancement was considered definite if it was thick, long or nodular, noted on greater than three contiguous images and extending deep into sulcal bases [1,2]. Meningeal enhancement was deemed equivocal if seen on less than three contiguous images or if it was not separately distinguishable from vascular enhancement. However, sulcal or subarachnoid spaces should normally show no enhancement on PCFLAIR sequence. Therefore, any enhancement along these spaces or cranial nerves was considered abnormal on this sequence [13].
Quantitative estimation was done by taking the single pixel signal intensities (SPSI) in the regions of meningeal or vascular enhancements. The SPSI were obtained in the exact region of interest (ROI) by placing a cursor at the same table positions using customized co-registration software in both pre and post contrast T1W with FS and FLAIR sequences and taking those automated mathematical values. The difference of the SPSI in meninges at basal cisterns and cortical sulci between pre and post contrast sequences was used to calculate the basal and leptomeningeal enhancements [Table/Fig-1a-d,2a-d]. Average of two measurements was taken. The average vascular enhancement was similarly calculated. The above two values were subtracted to obtain the net leptomeningeal enhancement. The statistical comparison of meningeal and vascular enhancement as derived by average SPSI in the region of interest between PCT1W with FS and PCFLAIR sequences was done.
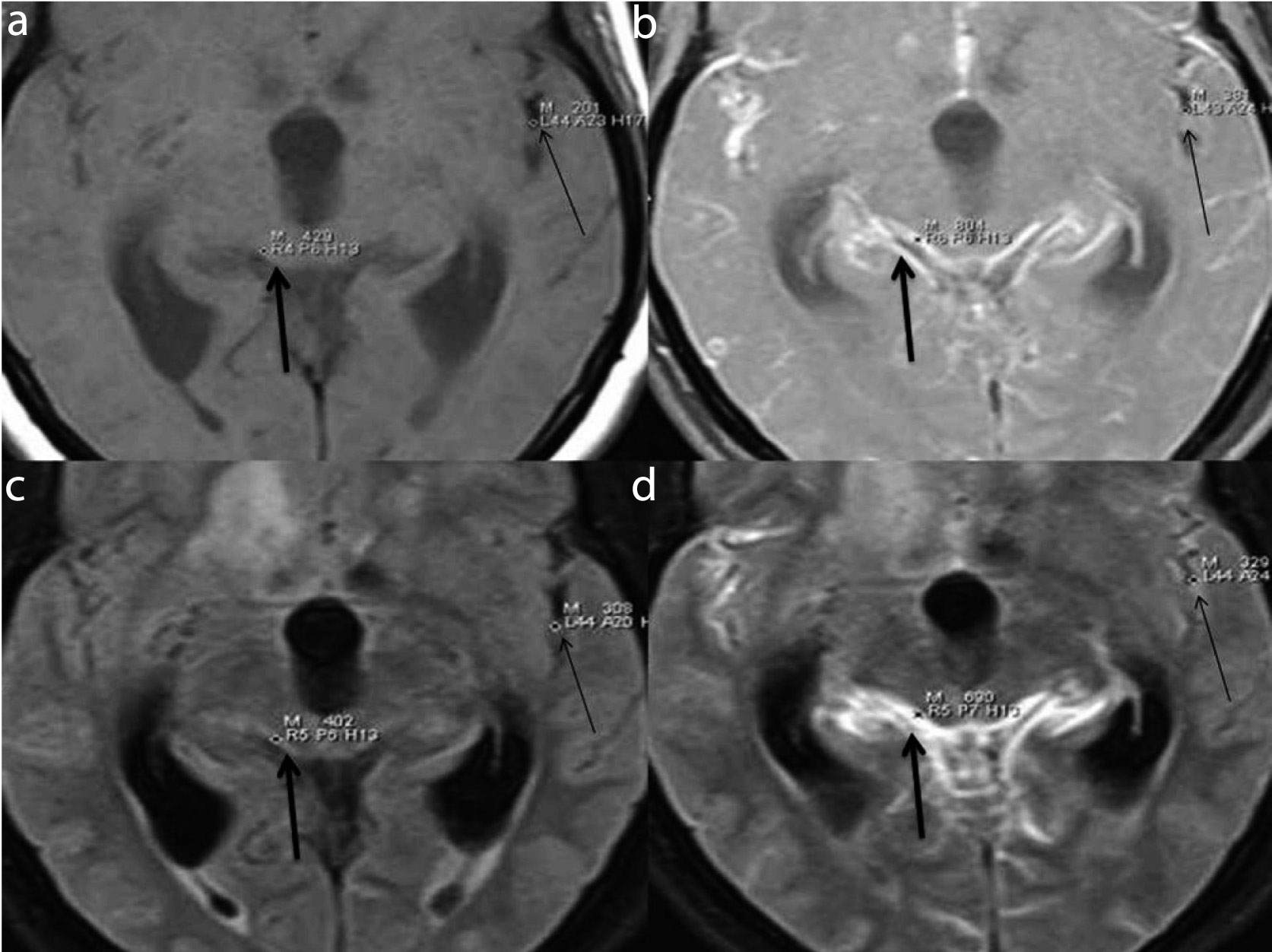
(a) Pre-contrast T1W with FS (b) Pre-contrast FLAIR (c) Post-contrast T1W with FS (D) Post-contrast FLAIR show quantitative estimation of meningeal enhancement (thick arrows) and vascular enhancement (thin arrows) by taking single pixel signal intensities in the regions of interest (cortical sulci and basal cisterns) in a case of viral meningitis. Images reveal insignificant vascular enhancement on PCFLAIR (d) making meningeal enhancement better appreciable.
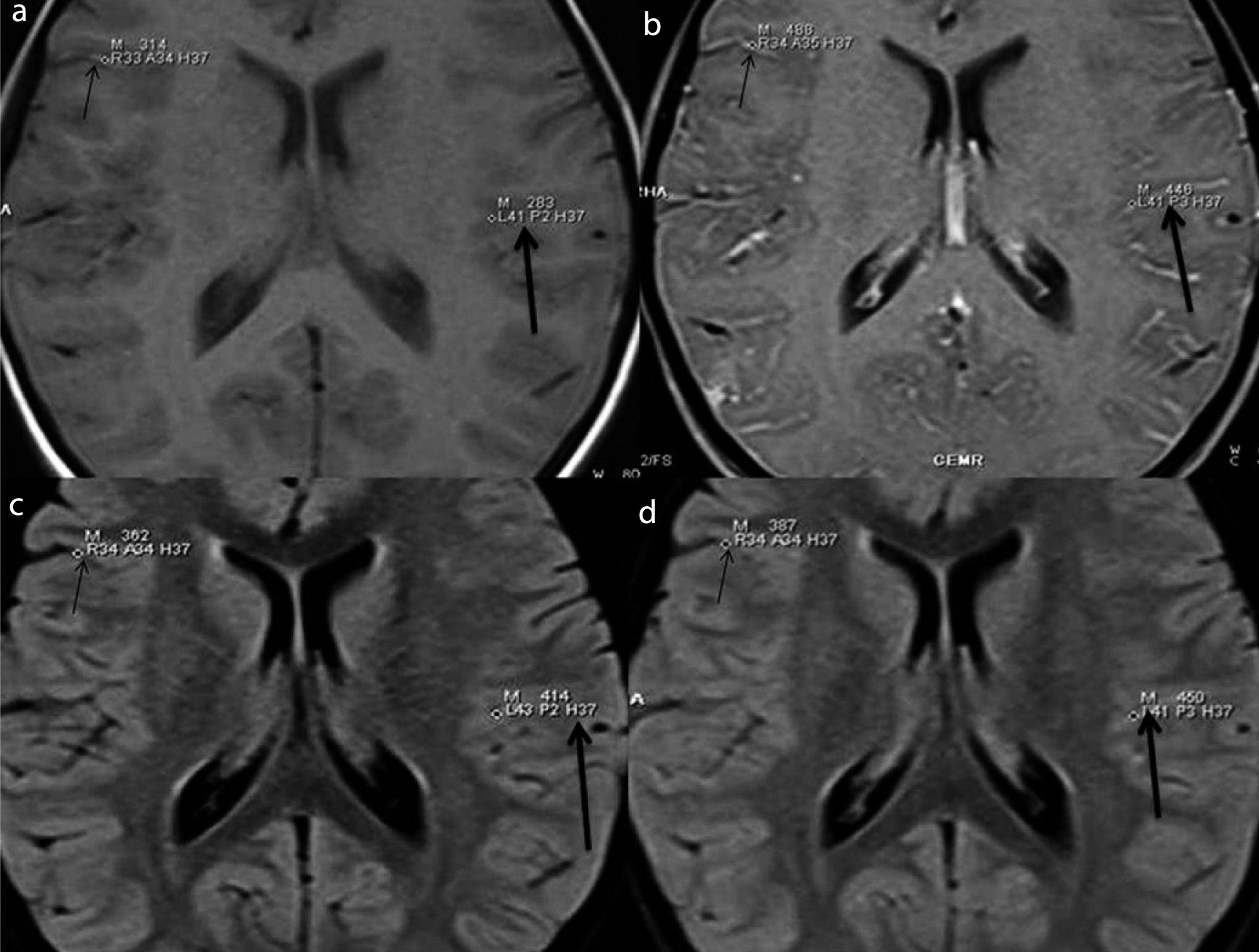
(a) Pre-contrast T1W with FS (b) Pre-contrast FLAIR (c) Post-contrast T1W with FS (d) Post-contrast FLAIR show quantitative estimation of meningeal enhancement (thick arrows) and vascular enhancement (thin arrows) by taking single pixel signal intensities in the regions of interest in a case of tubercular meningitis. Significant post contrast meningeal enhancement is seen on both sequences qualitatively. However, on PCFLAIR (d), better differentiation between meningeal and vascular enhancement is possible as there is hardly any vascular enhancement qualitatively and quantitatively.
Statistical Analysis
SPSS windows package was used for analysis. We compared the percentage accuracy of these sequences in qualitative differentiation of meningeal enhancement in various types of meningitis. The sensitivity and specificity was not estimated as the CSF culture is not possible in some cases of viral meningitis. An inter-observer reliability analysis using the kappa statistic was performed to determine consistency among observers. p-value of equal to or less than 0.05 was considered significant. Student t-test was used to ascertain the significance of differences between mean values of two continuous variables.
Results
This prospective study comprised of 31 patients (17 males and 14 females). Age of patients ranged from 4 years to 90 years (mean 47.51 ± 11.5 years). The clinical data is presented in [Table/Fig-3]. Qualitative assessment revealed definite meningeal signal even on pre-contrast FLAIR in 10 patients (7 tubercular, 3 pyogenic). On pre-contrast T1 with FS, meningeal signal could only be seen in 5 patients of tubercular meningitis. The overall qualitative accuracy of pre-contrast FLAIR in the detection of meningeal signal was 32.3% while the accuracy in tubercular and pyogenic meningitis was 47.6%. Pre-contrast T1 with FS had an overall accuracy of 16.1% only while the corresponding value in tubercular and pyogenic meningitis was 23.8%. None of the cases of viral meningitis demonstrated any meningeal signal on either of the pre-contrast sequences.
Clinical presentation, CSF analysis and diagnosis in the study population
| SN | Clinical Presentation | CSF Analysis |
|---|
| TLC | Po* | L* | Prot | Glu | GS | Z N | C/S | PCR | TR | D-Lac | ADA | Diagnosis |
|---|
| 1 | FV/NR | 4800 | 95 | 5 | 381.8 | 10 | N | N | P | | P | 15.4 | 2 | PM |
| 2 | FV/AS | 450 | 20 | 80 | 90 | 43 | N | N | ND | P | P | ND | 11.79 | TBM |
| 3 | NR/AS | 105 | 22 | 70 | 45 | 65 | N | N | ND | | P | ND | 2.7 | ViM |
| 4 | SZ/AS | 350 | 10 | 90 | 117 | 80 | N | N | ND | ND | P | ND | 31.4 | TBM |
| 5 | FV/HD | 110 | 24 | 74 | 71.5 | 64 | N | N | ND | | P | 2.3 | ND | ViM |
| 6. | FV/FND/AS | 1110 | 90 | 10 | 289 | 26 | N | N | P | | P | 13.5 | 1.8 | PM |
| 7 | FV/AS | 825 | 5 | 95 | 125 | 10 | N | N | N | P | P | ND | 18.2 | TBM |
| 8 | NR/AS | 1750 | 30 | 70 | 189 | 47 | N | N | ND | ND | P | ND | 10.41 | TBM |
| 9 | FV/HD | 90 | 32 | 68 | 38.5 | 54 | N | N | ND | | P | 1.8 | ND | ViM |
| 10 | FV/AS/FND | 250 | 17 | 83 | 162 | 44 | N | N | ND | ND | P | ND | 28.4 | TBM |
| 11 | FV/HD | 125 | 26 | 74 | 50 | 51 | N | N | ND | | P | ND | 2.3 | ViM |
| 12 | AS/HD | 120 | 18 | 82 | 56 | 68 | N | N | ND | | P | ND | ND | ViM |
| 13 | SZ/AS/HD | 405 | 26 | 71 | 128 | 43 | N | N | ND | ND | P | ND | 26.7 | TBM |
| 14 | NR/SZ | 200 | 28 | 72 | 197 | 51 | N | N | ND | P | P | ND | 17.89 | TBM |
| 15 | FV/AS | 240 | 13 | 87 | 119 | 47 | N | N | ND | ND | P | ND | 29.43 | TBM |
| 16 | NR/AS/HD | 520 | 14 | 85 | 97 | 39 | N | N | ND | ND | P | ND | 38.1 | TBM |
| 17 | FV/HD | 120 | 26 | 74 | 59 | 71 | N | N | ND | | P | ND | ND | ViM |
| 18 | AS/HD | 110 | 32 | 68 | 65 | 58 | N | N | ND | | P | ND | 0.81 | ViM |
| 19 | FV/NR/HD | 560 | 37 | 63 | 332 | 37 | N | N | P | ND | P | ND | 19.33 | TBM |
| 20 | AS/HD | 95 | 25 | 72 | 32 | 74 | N | N | ND | | P | ND | ND | ViM |
| 21 | NR/SZ/AS | 360 | 17 | 83 | 219 | 55 | N | N | ND | ND | P | ND | 30.4 | TBM |
| 22 | FV/NR/AS | 200 | 85 | 15 | 158 | 10 | N | N | P | | P | 8.6 | 3 | PM |
| 23 | AS/NR | 335 | 19 | 79 | 244 | 68 | N | N | N | P | P | ND | 33.4 | TBM |
| 24 | NR/AS/SZ | 470 | 5 | 95 | 117.8 | 44 | N | N | ND | ND | P | ND | 14.9 | TBM |
| 25 | SZ/NR | 1400 | 96 | 04 | 210 | 10 | N | N | ND | | P | 14.4 | 1.1 | PM |
| 26 | AR/NR/HD | 105 | 23 | 74 | 45 | 50 | N | N | P | | P | ND | ND | ViM |
| 27 | FV/AR | 1510 | 93 | 5 | 281 | 29 | N | N | N | | P | 12.8 | 2.6 | PM |
| 28 | FV/NR/HD | 600 | 12 | 88 | 318.4 | 46 | N | N | P | P | P | ND | 15.2 | TBM |
| 29 | FV/NR | 130 | 30 | 70 | 90 | 64 | N | ND | ND | | P | 1.3 | ND | ViM |
| 30 | FV/NR/HD | 1130 | 78 | 21 | 280 | 28 | P | ND | ND | | P | 11.3 | ND | PM |
| 31 | FV/SZ | 415 | 15 | 85 | 137.4 | 39 | N | N | N | N | P | ND | 22.5 | TBM |
FV=Fever Glu=Glucose (mg/dl); NR=Neck rigidity; GS=Gram Staining; AS=Altered Sensorium; ZN=Zeil Nelson staining; SZ=Seizure; HD= Headache C/S=Culture / Sensitivity
FND=Focal neurological deficit; TR=Therapeutic response; TLC=Total leukocyte count/μL; D-Lac=D-Lactate levels (mmol/L); Po = Polymorphs(%) ADA levels-Adenosine deaminase levels (IU/L); L=Lymphocytes (%); Prot=Proteins (mg/dl) PCR-Polymerase chain reaction for AFB
P- Positive N- Negative, ND- not done
TBM- Tubercular meningitis ViM- Viral meningitis, PM- Pyogenic meningitis
*Rest of cells were mainly monocytes
On PCFLAIR, appreciable meningeal enhancement was seen in 28 cases and vascular enhancement was not seen in any of these cases. On PCT1W with FS, we could separately delineate meningeal enhancement from vascular enhancement only in 17 cases, in 4 cases it was equivocal while in 10 cases of viral meningitis we could not separately differentiate abnormal meningeal enhancement.
The overall qualitative accuracy was 90.3% (28/31) for PCFLAIR sequence compared to 54.8% (17/31) for PCT1W with FS sequence. The qualitative accuracy of PCFLAIR in cases of tubercular and pyogenic meningitis was 100% (21/21) while the accuracy in viral meningitis was 70% (7/10). The corresponding accuracy for PCT1W with FS in demonstrating unequivocal meningeal enhancement was only 76.2% (17/21) for tubercular and pyogenic meningitis while we could not appreciate meningeal enhancement in any of the cases of viral meningitis. Both observers rated post-contrast FLAIR better than PCT1W at detecting meningitis (p < 0.05).
The statistical comparison of meningeal and vascular enhancement as derived by average SPSI in the region of interest between PCT1W with FS and PCFLAIR sequences are shown in [Table/Fig-4,5]. The quantitative assessment showed that the average SPSI of meningeal and vascular enhancements on PCT1W with FS sequence was greater as compared to PCFLAIR sequence (p<0.05). However, the net leptomeningeal enhancement on PCFLAIR was significantly greater than that on PCT1W with FS sequence (t= 6.31, p<0.01). In tubercular meningitis, the average basal enhancement (142.92±117) was significantly more than leptomeningeal enhancement (41.23±40.19) and the difference was statistically significant (t = 9.59, p<0.01)
Quantitative assessment of meningeal and vascular enhancement on PCT1W with FS and FLAIR sequences by single pixel signal intensities (SPSI) in the region of interest
| | Mean±S.D |
|---|
| 1. | Average T1 meningeal enhancement (Av MT1) | 155.91±76.31 |
| 2. | Average T1 vascular enhancement (Av VT1) | 192.57± 60.41 |
| 3. | Average meningeal T1-vascular T1 enhancement (Av (M-V) T1) | -36.19± 86.95 |
| 4. | Average meningeal enhancement FLAIR (Av M FLAIR) | 106.48± 67.07 |
| 5. | Average vascular enhancement FLAIR (Av V FLAIR) | 17.33± 13.32 |
| 6. | Average meningeal FLAIR -vascular FLAIR (Av (M-V) FLAIR) | 89.14± 61.63 |
Statistical comparison of meningeal and vascular enhancement on PCT1W with FS and FLAIR
| S. No. | Parameters | t-value | p-value | Interpretation |
|---|
| 1. | Av MT1 and Av VT1 | 1.9 | >0.05 | Not significant. Average meningeal enhancement on T1 is not significantly different from vascular enhancement. |
| 2. | Av MT1and Av M FLAIR | 2.32 | <0.05 | Significant. Average meningeal enhancement on T1 is significantly more than average meningeal enhancement on FLAIR |
| 3. | Av V T1 and Av V FLAIR | 12.14 | <0.001 | Significant. Average vascular enhancement on T1 is significantly more than average vascular enhancement on FLAIR |
| 4. | AV M FLAIR and Av V FLAIR | 6.62 | <0.001 | Significant. Average meningeal enhancement on FLAIR is significantly more than average vascular enhancement |
| 5. | Av (M-V) FLAIR and Av(M-V) T1 | 6.31 | <0.001 | Significant. Difference of average meningeal and vascular enhancement on FLAIR is significantly more than on T1. |
Discussion
Early detection of infectious meningitis is important for a favorable clinical outcome. CSF examination remains the gold standard for its diagnosis for which a lumbar puncture, an invasive procedure is mandatory. Imaging studies like MRI have been found to be helpful in detection of infectious meningitis especially in settings of viral and tubercular meningitis as CSF studies can be either non-contributory or take a longer time to get culture results. Moreover, we can monitor the complications of infectious meningitis using MRI [3,11,13].
Pre-contrast sequences can demonstrate meningeal signal (thickening) in only some cases of infectious meningitis. In the appropriate clinical settings, the MRI feature suggestive of meningitis is enhancement of leptomeninges after contrast administration. In normal meninges, the enhancement is subtle which appears thin and discontinuous and is prominent at parasagittal location. Abnormal meningeal enhancement is usually asymmetrical, thick, long and nodular, noted on more than three contiguous images and extends deep into the sulcal bases [3,10,11].
Spin echo T1-weighted sequence has been performed routinely after administration of Gadolinium based MRI contrast agents through intravenous route to detect the meningeal or parenchymal enhancement. These agents predominantly work by shortening the T1 relaxation time of protons located nearby which leads to enhancement of tissues. Other sequences have also been attempted to detect contrast enhancement in various meningeal pathologies like Proton density, T1-weighted sequence with MT saturation, T1-weighted with fat suppression and FLAIR including 3D FLAIR sequence [3,4–18]. Sequences based on T1-weighting have various limitations like too much vascular enhancement, flat images, and inflow effects [19]. Though FLAIR is a long TE sequence, the exact mechanism of post contrast enhancement may be partly due to the relaxivity (T1 shortening) effect, MT saturation effect or possibly due to yet unexplained mechanisms [7, 9, 11,13,14]. In extra axial lesions like meningitis or meningeal carcinomatosis, the post contrast enhancement is due to meningeal inflammation and increased vascularity. Normally slow flowing vessels would lead to appreciable hyperintensity in some of the sulcal spaces on pre-contrast T1WI. In meningitis, the vascular congestion is significantly increased. Therefore, separate distinction of vascular from adjacent meningeal enhancement may be less discernible. The FLAIR sequence offers a distinct advantage in overcoming the above limitations of T1WI. The signal from slow flowing vessels is suppressed on FLAIR due to lack of inflow enhancement phenomenon. Thus, any linear enhancement appreciable in the cortical sulci on PCFLAIR sequence is likely due to enhancing meninges rather than the adjacent enhancing vessels. In addition, CSF nulling effect allows for a better definition and superior delineation of meningeal enhancement [1,7,11,13,14].
In concordance with a previous report by Kremer et al., [12], we have also used qualitative accuracy as a statistical parameter to compare the FLAIR and T1W sequences in detection of infectious meningitis. The sensitivity and specificity was not estimated as the CSF culture is not possible in some cases of viral meningitis.
As reported previously, pre-contrast sequences were limited in their role for detection of infectious meningitis in our study. However, pre-contrast FLAIR had better accuracy compared to pre-contrast T1 in cases of tubercular and pyogenic meningitis. Detection of meningeal signal on pre-contrast FLAIR and T1 sequences is related to high CSF protein and would almost entirely rule out viral meningitis [3,13].
Postcontrast FLAIR sequence was acquired after PCT1W with FS sequence to study the effect of delayed leakage of contrast into subarachnoid space. In our study, the overall accuracy in the detection of meningeal enhancement in all the cases of infectious meningitis was 90.3% for PCFLAIR sequence compared to only 54.8% for PCT1W with FS sequence. We further observed that meningeal enhancement in cases of tubercular and pyogenic meningitis was appreciated readily on both PCFLAIR (100%) and PCTIW (76.2%) especially in region of basal cisterns. However, the SI from enhancing meninges in sulcal spaces along cerebral convexities is not unequivocally distinguishable from vascular enhancement on PCT1W, leading to the lower accuracy of PCT1W as also seen in other previous studies [3,12]. Better accuracy of PCFLAIR in infectious meningitis can further be explained by increased leakage of contrast from pial vessels into adjacent CSF from acutely inflamed meninges. Splendiani et al., [11] found the overall sensitivity of enhanced FLAIR sequence to be 100% while Parmar et al., [13] found it to be 85%. In the study by Kremer et al., [12], the accuracy in various leptomeningeal diseases including meningitis was 90%. Singh et al., [16] and Galassi et al., [18] have reported contrast enhanced PCTW1 with FS to be superior to contrast enhanced FLAIR imaging in leptomeningeal diseases. However, these studies were not limited to infectious meningitis and included other leptomeningeal pathologies like metastatic disease and chronic infections. Galassi et al., studied their patients by alternating FLAIR and PCTIW with FS as the first acquired sequence. In cases of metastatic disease and chronic infections, the meninges are both thickened and show increased vascularity. Contrast remaining in blood pool in such leptomeningeal diseases explains better enhancement and sensitivity of PCTIW in above studies while in infectious meningitis, both vascular congestion and subarachnoid leakage contribute to better enhancement on PCFLAIR [18].
A major challenge lies in the detection of subtle meningeal enhancement in cases of viral meningitis. Being a hill state, viral meningitis forms a substantial subset of patients referred to our institute. A few studies have described the importance of PCFLAIR in suspecting viral meningitis even before CSF cytology and cultures are available to the clinician but the strong distinctive comparative advantage of PCFLAIR (70% accuracy) compared to PCT1W (0% accuracy) in early detection of such cases has not been reported yet. The effectiveness of PCFLAIR in viral meningitis is also better than reported previously for post contrast magnetization transfer spin-echo T1 sequence. We found that the early detection of viral meningitis using PCFLAIR adds to the confidence of clinician and may lead to initiation of prompt therapeutic trial and better clinical prognosis. This may avoid empirical treatment of viral meningitis [3,11,13,20,21].
The possible elimination of vascular enhancement component on PCFLAIR has been observed previously [7,11,12,14]. However, to the best of our knowledge we have not come across any study which has made a quantitative evaluation of the vascular and meningeal components of enhancement in cases of meningitis. By using quantitative measurements, we have tried to statistically prove our qualitative observations regarding the relative advantages of PCFLAIR in detection of early and subtle cases of infectious meningitis especially those of viral origin. Vascular and meningeal enhancements on T1-weighted sequence were quantitatively proven to be significantly greater than that for FLAIR sequence due to T1 shortening effect. However, on FLAIR sequence, the net leptomeningeal enhancement was significantly greater compared to PCT1W. This is responsible for unequivocal visual appreciation of meningeal enhancement on PCFLAIR as observed qualitatively. It was also substantiated that in tubercular meningitis, the average basal enhancement was significantly more than leptomeningeal enhancement as reported previously [3,22].
The limitations of FLAIR sequence are related to slight increase in duration of the MR study by about one minute. Longer effective TE and CSF flow artefacts can sometimes lead to hyperintense sulci especially in children on pre-contrast FLAIR and may make distinction of post contrast meningeal enhancement difficult. Using a lower TE in our study eliminated these shortcomings and shortened the duration of study. Occasionally, the high signal in subarachnoid space is found in different conditions other than meningitis like subarachnoid hemorrhage, stroke, in patients who have received supplemental oxygen, gadolinium or iodinated contrast in the previous week or general anesthesia [15,16,22–24]. Such cases were excluded from our study and can even be ruled out by appropriate clinical evaluation. Simple SPSI may have some intra and inter-observer variations. We tried to minimize it by using customized co-registration software so that SPSI at same table positions are obtained. Since SPSI was just used to substantiate the qualitative observations, use of this tool did not have any major disadvantage in the present study. As a part of single imaging study protocol, it was not possible to compare PCFLAIR with various other modifications of conventional T1 sequences like post-contrast T1 magnetization transfer imaging [21]. However, this sequence also has the same limitations of T1- weighted sequences described earlier [18,25]. In addition, using 3D FLAIR instead of 2D FLAIR may reduce CSF flow artifacts [17] and improve the specificity of detection of meningeal enhancement. However, 3D FLAIR is not routinely available on all scanners and substantially increases the imaging time also. Multi-institutional trials with larger study population are required to develop the best imaging protocol in infectious meningitis.
Conclusion
PCFLAIR sequence is a very useful sequence in early detection of infectious meningitis especially of viral etiology due to its ability to unequivocally differentiate meningeal enhancement from vascular enhancement.
FV=Fever Glu=Glucose (mg/dl); NR=Neck rigidity; GS=Gram Staining; AS=Altered Sensorium; ZN=Zeil Nelson staining; SZ=Seizure; HD= Headache C/S=Culture / Sensitivity
FND=Focal neurological deficit; TR=Therapeutic response; TLC=Total leukocyte count/μL; D-Lac=D-Lactate levels (mmol/L); Po = Polymorphs(%) ADA levels-Adenosine deaminase levels (IU/L); L=Lymphocytes (%); Prot=Proteins (mg/dl) PCR-Polymerase chain reaction for AFB
P- Positive N- Negative, ND- not done
TBM- Tubercular meningitis ViM- Viral meningitis, PM- Pyogenic meningitis
*Rest of cells were mainly monocytes