Introduction and Aim: Type 2 diabetes mellitus (T2DM) is the predominant form of diabetes worldwide and much is known about its patho-physiology. Yet, newer aspects related to it are being constantly explored. For ages, testosterone has been known to men as the male sex hormone but now it has been shown by certain studies that it might have a role in the development of metabolic disorders like type 2 diabetes. This study was carried out to determine the relation of testosterone levels with type 2 diabetes mellitus and lipid profile in North East Indian men aged 31 to 73 years.
Materials and Methods: This case control study comprised of 40 type 2 diabetic men and 40 age matched non diabetic healthy men. Testosterone, SHBG levels and lipid profile were evaluated in both the groups along with anthropometric measurements and were statistically analysed.
Results: Serum total and free testosterone and Sex Hormone Binding Globulin were significantly lower in the test group than in the control group. Prevalence of type 2diabetes was five times higher in men having a total testosterone less than 8nmol/L and 5.57 times higher in those having a free testosterone of less than 0.225nmol/L. Fasting blood glucose showed a strong negative correlation with total and free testosterone. Glycated haemoglobin correlated negatively with SHBG but no such correlation was seen with total or free testosterone. Serum total and LDL cholesterol showed significant negative correlation with total testosterone and SHBG but no significant correlation was found with free testosterone. Serum VLDL, HDL and triglycerides did not show any significant correlation with total or free testosterone and SHBG levels.
Conclusion: Low testosterone might have a role in the development of type 2 DM and to the associated altered lipid profile. This study, though a small one is among the few of its kind in India and it thrives to assist other studies related to the matter.
Dyslipidemia, Serum total testosterone, Serum free testosterone, Sex hormone binding globulin, Type 2 diabetes
Introduction
Type-2 diabetes which accounts for approximately 90% of diabetes cases is a bipolar disease [1] characterized by impaired insulin action and abnormal secretion. The role of insulin is colossal in regulating glucose homeostasis through a highly orchestrated constellation of effects, which include promoting glucose uptake in peripheral tissues such as muscle and fat, suppressing hepatic glucose output, and regulating lipid metabolism [2]. In addition to insulin, hormones like glucagon, growth hormone, cortisol, catecholamines, insulin like growth factor-1 also have a role in maintaining glucose homeostasis [3]. In the recent years, androgen deficiency has captured the interest of many medical researchers and they have associated testosterone not only with the general health of men but also with certain common systemic illnesses, type 2 diabetes being one of them.
In general, most cross-sectional studies have reported higher endogenous testosterone concentrations to be associated with more favorable cardiovascular profile, including higher HDL cholesterol and lower triglyceride concentrations, blood glucose, blood pressure and body mass index. But, high doses of exogenous testosterone or other anabolic steroids have been associated with adverse health outcomes, including sudden cardiac death and hepatic disease [4]. Various debates have arisen whether low testosterone is involved in the pathogenesis of diabetes or it is merely a biomarker coexisting with diabetes. There is a dearth of research in this field in India, and more so in northeast India. Therefore, the objective of the present study is to evaluate the serum levels of testosterone in type 2 diabetic men and find any significant correlation between the different parameters.
Materials and Methods
The present study was carried out in the Department of Biochemistry of Gauhati Medical College and Hospital, Guwahati from May, 2012 to June, 2013. This work has been approved by the Institutional Ethics Committee, Gauhati Medical College, 2012. This case control study was conducted in a study population comprising of 80 male subjects with age ranging from 31 to 73 years who sought health care in the endocrinology and medicine outpatient departments in Gauhati Medical College and hospital and also the male attendants who accompanied them. Of these 80 subjects, 40 were type 2 diabetic men and 40 were healthy non diabetic men. Subjects with documented hypogonadism, liver failure, renal failure, those with any history of acute illness, alcohol consumption or smoking and those under sex hormone replacement therapy or any medication (e.g., diuretic, statins) that could have an influence on lipid metabolism, blood pressure, blood glucose, or body composition were excluded from the study. Along with detailed history, anthropometric measurements like body mass index and waist to hip ratio were also taken.
Taking all aseptic and antiseptic precautions, 5ml of blood was drawn from the median cubital vein. Fasting samples were used for all the investigations. Separated serum was used and tests were done within eight hours of collection, or else the samples were preserved at -20°C for future use. Estimations of serum fasting glucose [5], total cholesterol [6], triglyceride [7] and HDL [8] and glycated haemoglobin [9] were done using MERCK microlab 300 Semiautoanalyser. VLDL and LDL were calculated using Friedwald’s formula [10]. Estimations of serum total testosterone [11] and serum SHBG [12] were done using ELISA Microplate Reader (Biorad 680). Serum free testosterone [13] was calculated by the free testosterone calculator using serum total testosterone, SHBG and albumin based on the Vermeulen’s formula. After all the calculations and the biochemical estimations, the results obtained were statistically analysed and compared between different groups of the study. Baseline characteristics of the study participants are expressed in mean ± SD. Mann Whitney U-test, student t-test and Fisher’s exact test were used whenever applicable to analyse differences in baseline characteristics between the control and the test groups. Correlations were observed using Spearmen correlation coefficient. The results were considered significant when the probability (p-value) was less than 0.05 % of the observed values of “t” at a particular degree of freedom. Statistical analysis was done using Graph Pad InStat version 3.00. All the statistical graphs were prepared using Microsoft Excel 2007.
Ethics
The study was approved by the Institutional Ethics Committee of Gauhati Medical College & Hospital, Guwahati and the ethical clearance number was MC/90/2012/Pt-11/16.
Results
The control group comprised of 40 men with age ranging from 31 to 71. Most of the control subjects were in the age group 51-60 y with a relative frequency of 0.37. The test group also comprised of 40 individuals with age ranging from 32 to 73 and here also most of the subjects were in the age group 51 to 60 y with a relative frequency of 0.37.
Our results from [Table/Fig-1] shows the comparison of anthropometric and biochemical characteristics between the case and control groups. Men in the test group had significantly higher BMI, waist circumference, WHR, Systolic and diastolic B.P, FBG, total cholesterol, LDL cholesterol, VLDL cholesterol, triglycerides and glycated haemoglobin. There was no significant difference between the HDL cholesterol of the two groups. Serum total testosterone (TT), calculated free testosterone (cFT) and SHBG were significantly lower in the test group than in the control group.
Comparison of means of the different anthropometric, clinical and biochemical characteristics between the test and control groups
| Parameters | Control group | Diabetic group | p-value |
|---|
| Age (Years) | 53.37±10.34 | 57.52±9.17 | 0.061 |
| BMI (Kg/m2) | 23.42±2.250 | 25.598±2.148 | <0.0001 |
| Waist circumference (cm) | 75.62±10.54 | 82.82±10.38 | 0.0029 |
| WHR | 0.88±0.05 | 0.94±0.05 | <0.0001 |
| Sys B.P (mm Hg) | 129.75±15.68 | 140.70±20.03 | 0.0072 |
| Diastolic B.P (mm Hg) | 81.45±7.002 | 85.500±9.389 | 0.0222 |
| FBG (mg/dL) | 89.17±12.92 | 180.35±77.24 | <0.0001 |
| Total cholesterol (mg/dL) | 154.1±35.05 | 193.50±48.99 | <0.0001 |
| HDL-Cholesterol (mg/dL) | 41.25±8.44 | 38.2±14.43 | 0.2522 |
| LDL-Cholesterol (mg/dL) | 89.45±28.83 | 126.70±51.25 | 0.0004 |
| VLDL-Cholesterol (mg/dL) | 23.40±7.53 | 29.67±12.2 | 0.023 |
| Triglyceride (mg/dL) | 117±37.68 | 148.38±61.00 | 0.023 |
| Glycated haemoglobin (%) | 5.32±0.56 | 8.07±2.54 | <0.0001 |
| Total testosterone (nmol/L) | 18.55±8.29 | 10.20±9.23 | <0.0001 |
| Serum SHBG (nmol/L) | 61.99±29.04 | 39.18±31.91 | <0.0001 |
| Free testosterone (nmol/L) | 0.293±0.163 | 0.217±0.258 | 0.0051 |
[Table/Fig-2] shows that the prevalence of type 2 diabetes is significantly more in men with a total testosterone of less than 8nmol/L. An increased risk of type 2 diabetes by 5 times(1.91-13.06, 95% confidence interval and p= 0.0014) was observed in those having total testosterone levels less than 8 nmol/L. Similarly, an increased risk of type 2 diabetes by 5.571 times (2.11-14.65, 95% confidence interval and p= 0.0006) was seen in men with free testosterone less than 0.225nmol/L.
Prevalence of low testosterone levels in type 2 diabetic men
| Status of serum testosterone levels | Total cases | Type 2 DM present | Type 2 DM absent | Odds ratio | 95% confidence interval | p-value |
|---|
| Frequency (percentage) | Frequency (percentage) |
|---|
| TT≤8nmol/L | 35 | 25(31) | 10(13) | 5 | 1.91-13.06 | 0.0014 |
| FT≤.225nmol/Ls | 44 | 30(38) | 14(18) | 5.571 | 2.11-14.65 | 0.0006 |
As seen in [Table/Fig-3], there is a strong negative correlation of age with both total and free testosterone, whereas its correlation with SHBG is not significant. There was no significant correlation of BMI with total and free testosterone and with SHBG. There was a strong negative correlation of WHR with both total and free testosterone but not with SHBG. FBG showed a strong negative correlation with total and free testosterone but that with SHBG was not so strong. Glycated haemoglobin correlated negatively with SHBG but no such correlation was seen with total or free testosterone. Serum total cholesterol and LDL cholesterol showed significant negative correlation with total testosterone and SHBG but no significant correlation was found with free testosterone. Serum VLDL, HDL and triglycerides did not show any significant correlation with total or free testosterone and also with SHBG levels.
Correlation of serum total, free testosterone and SHBG with different parameters in the test group
| Parameters | Serum total testosterone | Serum free testosterone | Serum SHBG |
|---|
| Correlation coefficient | p-value | Correlation coefficient | p-value | Correlation coefficient | p-value |
|---|
| AGE | -0.67 | <0.0001 | -0.59 | <0.0001 | -0.22 | 0.1694 |
| BMI | -0.207 | 0.198 | -0.176 | 0.2767 | 0.05 | 0.7557 |
| WHR | -0.447 | 0.0038 | -0.411 | 0.0084 | -0.03 | 0.8403 |
| FBG | -0.49 | 0.0012 | -0.41 | 0.0076 | -0.27 | 0.083 |
| Glycated haemoglobin | -0.26 | 0.0969 | -0.12 | 0.44 | -0.33 | 0.0342 |
| Serum cholesterol | -0.36 | 0.0212 | -0.205 | 0.2057 | -0.43 | 0.0049 |
| Seum LDL-cholesterol | -0.37 | 0.0162 | -0.195 | 0.2256 | -0.47 | 0.0021 |
| Serum VLDL-cholesterol | -0.03 | 0.84 | -0.008 | 0.9567 | -0.18 | 0.255 |
| Serum HDL-cholesterol | 0.1 | 0.52 | 0.01 | 0.94 | 0.27 | 0.0856 |
| Serum triglycerides | -0.03 | 0.84 | -0.008 | 0.95 | -0.33 | 0.0342 |
Discussion
In our study, we found that in the test group maximum patients were of the age group 51-60 y. The mean age of the diabetic group was 52.57 y which was not significantly higher than that of the control group (p=0.061). The test group which comprised of diagnosed type 2 diabetic patients had mean BMI of 25.59 Kg/m2 and WHR of 0.94 which were significantly higher than in the control group with a p-value of <0.0001 in both the cases as seen in [Table/Fig-1]. This finding corroborates with the findings of Chandel et al., [14] and Shah et al., [15]. As per Mukhtar et al., [16] visceral obesity reflected in the raised WHR might be attributed to insulin resistance which is a characteristic of type 2 diabetes. Both systolic and diastolic BP were significantly higher in the test group with p values of 0.007and 0.02 respectively. Most type 2 diabetic men are often hypertensive during diagnosis. And this hypertension has characteristics suggestive of hypertension of the elderly [17].
The mean fasting serum glucose in the test group and control group were 180.35mg/dL and 89.17mg/dL respectively. As expected, fasting serum glucose was significantly higher in the test group with a p-value of <0.0001 which corroborates with the finding of Abate et al., [18]. Glycated haemoglobin too was significantly higher in the test group with a p-value of <0.0001. This finding tallies with the findings of Zhang et al., [19].
The mean serum total cholesterol and the mean LDL cholesterol in the test group were 193.5 mg/dL and 126.7mg/dL respectively which were significantly higher than in the test group with p-values of <0.0001 and 0.0004 respectively. These findings corroborate with the findings of Samatha et al., [20]. The mean VLDL cholesterol in the control group was 23.4mg/dL which was significantly lower than that of the test group with a mean of 29.67mg/dL (p=0.023). The mean triglyceride level in the control group was 117mg/dL which was significantly lower than in the test group with a mean of 148.38mg/dL (p=0.023). Type 2 diabetes is often associated with dyslipidemia and therefore increases cardiovascular risks. Altered metabolism of triglyceride rich lipoproteins plays a pivotal role in development of this dyslipidemia. Alterations include both increased hepatic secretion of VLDL and impaired clearance of VLDL and intestinally derived chylomicrons. This increased VLDL results in rise of small dense LDL particles [21].
Mean serum total testosterone (TT) of the non diabetic group was 18.88 nmol/L which was significantly higher than that of the diabetic group (10.20nmol/L) with a p-value of <0.0001 and this observation is comparable to that of Adrekani et al., [22]. Low levels of testosterone are linked with insulin resistance and implicated in hyperglycemia, hypertension, dyslipidemia and an increased risk of vascular diseases [23]. Calculated FT (Mean=0.293nmol/L) of the control group was found to be significantly higher than that of the test group (Mean=0.217nmol/L) with a p-value of 0.0051. This finding is in accordance with that of Haffner et al., [24]. But, Andersson et al., [25] did not find any difference of FT between the 2 groups. In our study, 30(38%) of the subjects having cFT less than 0.225nmol/L suffered from DM as depicted in [Table/Fig-2], whereas, only 10(13%) of the study group having cFT >0.225nmol/L were afflicted by type 2 DM. The odds ratio was 5.571 and the p value was extremely significant (p=0.0006). This is in accordance with the findings of Kapoor et al., [26].
The mean serum SHBG of the control group was 61.99nmol/L whereas that of the test group was 39.18nmol/L. The difference between the two groups was extremely significant with a p-value of <0.0001 and this finding is in accordance with that of Andersson et al., [25] and Haffner et al., [24]. SHBG is a major determinant of clearance of sex hormones and regulates the availability of active testosterone at target tissues. Recent molecular epidemiologic studies have shown that genetically determined levels of SHBG were inversely associated with the risk of type 2 DM, lending support to the role of SHBG in its development [27].
Age was found to be inversely correlated with Serum TT and cFT. And the correlation was extremely significant with p-values of < 0.0001 as depicted in [Table/Fig-3]. This finding is in accordance with that of Vikan et al., [28]. Serum SHBG also negatively correlated with age but the correlation was not significant. BMI correlated inversely with TT, cFT and SHBG but the correlations were not significant. But, WHR correlated inversely with TT and cFT in a significant manner (r=-0.44, p=0.0038; r=-0.41, p=0.0084 respectively). BMI is a measure of whole body obesity. In contrast, WHR is a measure of central obesity and has been found to be a major predictor of cardiovascular diseases [29]. This visceral obesity leads to increased break down of testosterone to estrogen by the action of aromatase. This estrogen in turn suppresses the release of GnRH and LH thereby further decreasing the levels of testosterone [30].
As shown in [Table/Fig-3], FBG correlated inversely with both TT and cFT(r=-0.49, p=0.0012; r=-0.41, p=0.0076 respectively); the association being stronger with TT. SHBG also correlated negatively with FBG but the correlation was not very significant. As per Ueshiba [31], the fasting blood glucose improved in men with metabolic syndrome after administration of testosterone in a Japanese male population. Thus, they concluded that testosterone administration improves insulin resistance. Low testosterone leads to a decrease in muscle mass and an increase in circulating free fatty acids [32]. Free fatty acids mediate the development of insulin resistance and ultimately the development of overt type 2 DM [33]. Also, it was seen that when non diabetic experimental rats were exposed to subtherapeutic or supratherapeutic doses of testosterone, they developed insulin resistance [32]. SHBG correlated significantly with glycated haemoglobin (r=-0.33, p= 0.03). But the correlation of TT and cFT with glycated haemoglobin was not significant. Chubb et al., [34] showed that SHBG is more strongly associated with metabolic syndrome in older men than TT. This suggests that association between TT and metabolic syndrome might be secondary to the association between SHBG and metabolic syndrome. As per Wang et al., [35], low TT and SHBG predict the development of diabetes independent of age, race and obesity and that SHBG was a stronger predictor of type 2 diabetes.
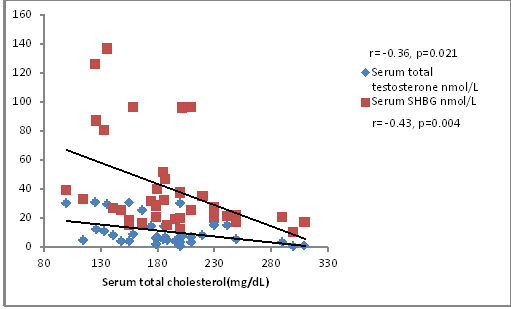
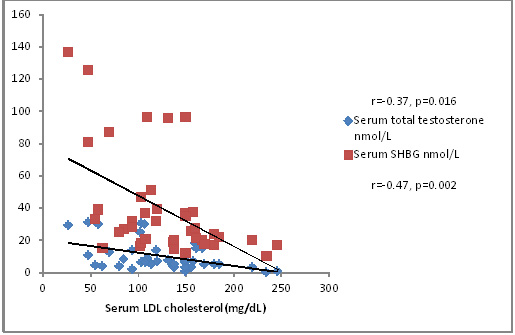
Total cholesterol correlated inversely with TT and SHBG levels (r=-0.36, p=0.021; r=-0.43, p=0.004) as depicted in [Table/Fig-4]. This finding is in accordance with that of Laaksonen et al., [36]. But this association was stronger with SHBG than with TT. Free testosterone also correlated inversely with total cholesterol levels but it was not significant. As shown in [Table/Fig-5], serum LDL cholesterol also correlated negatively with TT and SHBG levels (r=-0.37, p=0.016; r=-0.47, p=0.0021). VLDL cholesterol did not show any significant correlation with TT, cFT or SHBG levels. HDL cholesterol correlated with SHBG levels (r=0.27, p=0.08) but not very significantly. Serum triglycerides did not show any significant correlation with serum TT, cFT or SHBG levels. Low levels of testosterone is associated with visceral fat accumulation, metabolic syndrome, type 2 diabetes, increased inflammatory cytokines, hyperlipidemia and abnormalities of coagulation [35]. Epidemiological data suggest that testosterone levels are associated negatively with total cholesterol, LDL cholesterol and triglycerides, and positively with HDL cholesterol. Similarly, trials of testosterone replacement have shown improvement in the lipid profile of dyslipidemic men. But the exact mechanism as to how testosterone levels affect lipid profile is still not known. However, considering the enormous support on this possibility lend by various studies low testosterone can be regarded as a new cardiovascular risk factor in men [37].
Correlation of total cholesterol with total testosterone and SHBG
Correlation of LDL cholesterol with serum total testosterone and SHBG levels
Conclusion
This study showed that testosterone levels correlated inversely with blood sugar status in men, suggesting its role in the development of type 2 DM. Serum testosterone and SHBG levels were significantly lower in type 2 diabetic men and levels of total testosterone correlated inversely with total cholesterol and LDL cholesterol. This suggests that androgens affect lipid profile in a not very well known mechanism, thereby increasing CVD risk. To precisely establish the role of testosterone in type 2 diabetes, a more elaborate study with larger study group would have been desirable as our study was limited by a small study group. However, this was our earnest effort with whatever resources available to contribute towards men’s health.
[1]. Scheen AJ, Pathophysiology of type 2 diabetesActa Clinica Belgica 2004 58(6):335-41. [Google Scholar]
[2]. Xue B, Kim YB, Lee A, Toschi E, Weir SB, Kahn CR, Protein-tyrosine phosphatase 1B deficiency reduces insulin resistance and the diabetic phenotype in mice with polygenic insulin resistanceThe journal of biological chemistry 2007 282(33):23829-40. [Google Scholar]
[3]. Yeo RP, Sawdon M, Hormonal control of metabolism: regulation of plasma glucoseAnaesthesia intensive care medicine 2010 11(7):279-83. [Google Scholar]
[4]. Khaw KT, Dowsett M, Folkerd E, Bingham S, Wareham N, Luben R, Endogenous sex hormone and mortality due to all causes, cardiovascular disease, and cancer in men: European prospective investigation into cancer in Norfolk (EPIC-Norfolk) prospective population studyCirculation 2007 116(23):2694-701. [Google Scholar]
[5]. Lott JA, Turner K, Evaluation of Trinder’s Glucose Oxidase Method for Measuring Glucose in Serum and UrineClin Chem 1975 21(12):1754-60. [Google Scholar]
[6]. Rifai N, Bachorik PS, Albers JJ, Lipids, lipoprotein and apolipoprotein.In: Burtis CA, Ashwood R, editorsTietz textbook of clinical chemistry 1999 3rd edPhiladelphiaW.B. Saunders Company:806-61. [Google Scholar]
[7]. Bucolo G, David H, Quantitative determination of serum triglycerides by the use of enzymesClin Chem 1973 19:476-82. [Google Scholar]
[8]. Sugiuchi H, Uji Y, Okabe H, Irie T, Uekama K, Kayahara N, Direct measurement of High-Density Lipoprotein Cholesterol in serum with polyethylene glycol-modified enzymes and sulphated alpha-cyclodextrinClin Chem 1995 41:717-23. [Google Scholar]
[9]. Trivelli LA, Ranney HM, Lai HT, Hemoglobin components in patients with diabetes mellitusN Engl J Med 1971 284(7):353-57. [Google Scholar]
[10]. Friedewald WT, Levy RI, Fredrickson DS, Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifugeClin Chem 1972 18:499-502. [Google Scholar]
[11]. Marcus GJ, Dunford R, A simple linked immunoassay for testosteroneSteroids 1985 46:975-86. [Google Scholar]
[12]. Lewis JG, Longley NJ, Elder PA, Monoclonal antibodies to human SHBG: characterization and use in a simple ELISA of SHBG in plasmaSteroids 1999 64:259-65. [Google Scholar]
[13]. Vermeulen A, Verdonck L, Kaufman JM, A critical evaluation of simple methods for the estimation of free testosterone in serumThe journal of clinical endocrinology and metabolism 1999 84(10):3666-72. [Google Scholar]
[14]. Chandel A, Dhindsa S, Topiwala S, Choudhury A, Dandona P, Testosterone concentrations in young patients with diabetes mellitusDiabetes care 2008 162(4):747-54. [Google Scholar]
[15]. Shah A, Bhandari S, Malik SL, Risal P, Koju R, Waist circumference and waist hip ratio as predictors of type 2 diabetes mellitus in the Nepalese population of Kavre diatrictNepal Med Coll J 2009 11(4):261-67. [Google Scholar]
[16]. Al Mukhtar SB, Fadhil NN, Hanna BE, Serum lipid profile in subjects with type 2 diabetes mellitus and hypertension in relation to metabolic syndrome: a case control studyDuhok Medical Journal 2012 6(2):29-44. [Google Scholar]
[17]. Epstein M, Sowers JR, Diabetes mellitus and hypertensionHypertension 1992 19:403-18. [Google Scholar]
[18]. Abate N, Haffner SM, Garg A, Peshock RM, Grundy SM, Sex steroid hormone, upper body obesity and insulin resistanceThe Journal of Clinical Endocrinology and Metabolism 2002 87(10):4522-27. [Google Scholar]
[19]. Zhang HY, Wu CJ, Li CS, Glycated haemoglobin A1C and diabetes mellitus in critically ill patientsWorld J Emerg Med 2013 4(3):201-04. [Google Scholar]
[20]. Samatha P, Venkateswarlu M, Siva Prabodh V, Lipid profile levels in type 2 diabetes mellitus from the tribal population of Adilabad in Andhra Pradesh, IndiaJournal of Clinical and Diagnostic Research 2012 6(4):590 [Google Scholar]
[21]. Krauss RM, Lipids and lipoproteins in patients with type 2 diabetesDiabetes care 2004 27:1496-504. [Google Scholar]
[22]. Adrekani MA, Borgian L, Adrekani JM, Chiti Z, Rashidi M, Azod L, The evaluation of serum level of testosterone and sex hormone binding globulin in men with type 2 diabetesIranian Journal of diabetes and Obesity 2010 2(1):12-15. [Google Scholar]
[23]. Traish AM, Saad F, Guay A, The dark side of testosterone deficiency: Type 2 diabetes and insulin resistanceJournal of andrology 2009 30:23-32. [Google Scholar]
[24]. Haffner SM, Shaten J, Stern MP, Smith GD, Kuller L, Low levels of sex hormone binding globulin and testosterone predict the development of non insulin dependent diabetes mellitus in menAm J Epidemiol 1996 143:889-97. [Google Scholar]
[25]. Andersson B, Marin P, Lissner L, Vermeulen A, Bjorntorp P, Testosterone concentrations in women and men with NIDDMDiabetes care 1994 17(5):405-11. [Google Scholar]
[26]. Kapoor D, Aldred M, Clark R, Channer KS, Jones, TH, Clinical and biochemical assessment of hypogonadism in men mith type 2 diabetesDiabetes Care 2007 30:911-17. [Google Scholar]
[27]. Goto A, Morita A, Goto M, Sasaki S, Miyachi M, Aiba N, Assoiations of sex hormone binding globulin and testosterone with diabetes among men and wome (the Saku Diabetes study): a case control sudyCardiovascular diabetology 2012 11(130) [Google Scholar]
[28]. Vikan T, Schirmer H, Njolstad I, Svartberg J, Low testosterone and SHBG levels and high estradiol levels are independent predictors of type 2 diabetes in menEJE 2010 [Google Scholar]
[29]. Gupta R, Rastogi P, Sarna M, Gupta VP, Sharma SK, Kothari K, Body mass index, waist size, waist hip ratio, and cardiovascular risk factors in urban subjectsJapi 2007 55:621-27. [Google Scholar]
[30]. Mohr BA, Bhasin S, Link CL, O’Donnell AB, McKinlay JB, The effect of changes in adiposity on testosterone levels in older men: longitudinal results from the Massachusetts male aging studyEuropean journal of endocrinology 2006 155:443-52. [Google Scholar]
[31]. Ueshiba H, Testosterone treatment improves insulin resistance in Japanese male metabolic syndromeSteroids and hormonal change 2013 4(2) [Google Scholar]
[32]. Makhsida N, Shah J, Yan G, Fisch H, Shabsigh R, Hypogonadism and metabolic syndrome: implications for testosterone therapyThe journal of urology 2005 174:827-34. [Google Scholar]
[33]. Bhattacharya S, Dey D, Roy SS, Molecular mechanism of insulin resistanceJ Biosci 2007 32(2):405-13. [Google Scholar]
[34]. Chubb SAP, Hyde Z, Almeida OP, Flicker L, Norman PE, Zamrojik K, Lower sex hormone binding globulin is more strongly associated with metabolic syndrome than lower total testosterone in older men: the health in men studyEuropean journal of endocrinology 2008 158:785-92. [Google Scholar]
[35]. Wang C, Jackson G, Jones TH, Matsumoto AM, Nehra A, Perelman MA, Low testosterone associated with obesity and the metabolic syndrome contributes toSexual dysfunction and cardiovascular disease risk in men with type 2 diabetesDiabetes care 2011 34:1669-75. [Google Scholar]
[36]. Laaksonen DE, Niskanen L, Punnonen K, Nyyssonen K, Tuomainen TP, Salonen, R, Sex hormone, inflammation and the metabolic syndrome: a population based studyEuropean jounal of endocrinology 2003 149:601-08. [Google Scholar]
[37]. Maggio M, Basaria S, Welcoming low testosterone as a cardiovascular risk factorInternationalJournal of Impotence Research 2009 21:261-64. [Google Scholar]