Evaluation of Cytotoxicity of Silorane and Methacrylate based Dental Composites using Human Gingival Fibroblasts
Prashanthi Sampath Madhyastha1, Dilip G Naik2, Ravindra Kotian3, Divya Padma4, N Srikant5, Kumar M.R Bhat6
1 Senior Grade Lecturer, Department of Dental Materials, Manipal College of Dental Sciences, Manipal University, Mangalore, India.
2 Professor, Department of Periodontics, Manipal College of Dental Sciences, Manipal University, Mangalore, India.
3 Reader, Department of Dental Materials, Manipal College of Dental Sciences, Manipal University, Mangalore, India.
4 Research Assistant, Department of Anatomy, Kasturba Medical College, Manipal University, Manipal, India.
5 Associate Professor, Department of Oral Pathology, Manipal College of Dental Sciences, Manipal University, Mangalore, India.
6 Additional Professor, Department of Anatomy, Kasturba Medical College, Manipal University, Manipal, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Kumar M R Bhat, Additional Professor, Department of Anatomy, Kasturba Medical College, Manipal University, Manipal-576104, India.
E-mail: kummigames@yahoo.com
Aim: The effects of leached substances from the restorative dental materials may induce local and systemic adverse effects. Thus the biological and toxic properties of the restorative dental materials must be compatible with the oral tissues or with general health. Therefore, the need for biocompatible restorative dental material implies the necessity of toxicity testing. It was the purpose of this investigation to determine and compare the possible toxic effect of silorane based composite (Filtek P90) on human gingival fibroblast (HGF) in vitro using cytotoxicity measuring parameters (MTT assay) in comparison with its methacrylate counterpart (Z100) for their viability, proliferation rate.
Materials and Methods: Fresh healthy biopsy specimens of human gingival tissue of patients were obtained. For HGF, cells were cultured in Dulbecco’s modified Eagle medium and grown to sub confluent monolayers. After attaining confluence, cells were treated with different doses of the Filtek P90 or Z 100 for different time point. HGF cells were observed for their proliferation, viability by MTT assay.
Results: The results of the cytotoxicity assay showed that, the percentage of viable cells was very good in the first 24h and marginally decreased in the next 48h period in all groups. However, the proliferation rate was never below 84% in all the groups, at any given concentration. Filtek P90 and Z100 treated cells exhibited insignificant decrease in the cell proliferation both in 24h and 48h exposure when compared to significant decrease in the cell survival rate in the positive control (Mitomycin C 250 μg/ml).) Comparison of the toxicity between Filtek P90 and Z100 in 24h & 48h separately showed that there was no significant difference (p<0.05) between these two composites in 24h and 48h’ time period at all concentrations of the composites.
Conclusion: To conclude, the new silorane based restorative composite showed comparable cytotoxic characteristics to clinically successful dimethacrylate composites suggesting the non-toxic nature in the oral environment and hence contributing to clinical success of these new restorative materials.
Cytotoxicity, Human gingival fibroblasts, Methacrylate-based composites, MTT Assay, Silorane-based composites
Introduction
Restorative dental materials are placed in direct contact with living tissues of oral cavity. With time, the composition of the restorative materials changes due to chemical and mechanical degradation in the oral cavity. They may also influence the health of oral soft tissues in several ways especially by delivering water soluble components [1–3] into saliva/oral cavity as well as by interacting directly with adjacent tissues like epithelia of gingiva and its connective tissue [2]. It has been shown that, the organic matrix of the dental composite resins, when released into the oral cavity can cause a wide variety of adverse biological reactions such as mucosal irritation, epithelial proliferation, oral lichenoid reaction, hypersensitivity, anaphylactoid reactions and may also cause fibrosis of the adjacent soft tissue [2,4–6]. Thus the biological and toxic properties of the dental materials must be compatible with the oral tissues and even with general health. Therefore, the need for biocompatible restorative dental material implies the necessity of toxicity testing.
The importance of using cell culture models to test the biocompatibility of dental materials is well established [2,7] with the help of cultured fibroblast from human pulp, gingiva, skin, buccal mucosa, periodontal membrane, oral epithelium and commercially available cell lines such as HeLa cells, BALb/c 3T3 etc. Human gingival fibroblasts are most frequently used in the biological assessment of dental materials [2,8–10].
Methacrylate based dental composite has been widely used as filling (restorative) material in dentistry [11] which undergoes a free radical addition polymerization mechanism. Polymerization shrinkage and marginal integrity of restorations are the inevitable problems with methacrylate based composite [11]. Two to three percentage shrinkage in the material causes stress at tooth restorative interface leading to postoperative pain, fracturing of restoration, chipping of margins, debonding, microleakage. These inevitable problems caused by methacrylate composites can be minimized by changing the chemistry of setting and using silorane based composites which became highly desirable. This initiated the development of new innovation in the field of dental composites.
A silorane based composite (Filtek P 90), comprising of ring-opening monomer, claims low polymerization shrinkage (<1%). This low shrinkage is because of ring opening polymerization process of cyclic epoxides. It is desirable in the development of a new dental composite filling material superior to the current resin compounds based on acrylates [11–14]. Silorane contains siloxane and oxirane. Polymerization of silorane-based composite via a photocationic ring-opening reaction results in a lower polymerization contraction compared to methacrylate based composite. In addition to low polymerization shrinkage due to the oxirane ring-opening polymerization reaction [12,13] it also has hydrophobicity due to the presence of siloxane which promotes the insolubility of the material in the presence of oral fluids [15,16].
The biocompatibility of silorane based composite (Filtek P90) was tested using individual constituents of silorane rather than Filtek P90 as a whole. There is no report to show the sensitivity of oral fibroblasts harvested from patients on Filtek P90. Therefore, the present study is designed to evaluate and compare the cytotoxic effect of silorane based composite (Filtek P90) and methacrylate based counterpart (Z100) on human gingival fibroblast (HGF). Since the possible toxic effect can culminate in various oral health problems, the results of this test of Filtek P90 may suggest the compatibility of these materials in the oral environment and hence, may contribute to clinical success of this new material.
Materials and Methods
Preparation of stock solution: The two composites used in the study [Table /Fig-1] were dissolved in dimethylsulfoxide (DMSO). From the stock solution (10mg/ml), seven different concentration of composite (20, 10, 5, 2.5, 1.25, 0.625 and 0.3125 μg/ml) were used to treat cells at the final concentration of DMSO less than 0.2%.
Materials used in the study
| Name | Type | Manufacturer | Shade | Organic matrix | Inorganic fillers | Filler content [wt.%] |
|---|
| Z100 | Methacrylate based universal composite | 3M/ESPE, St. Paul, MN, USA | A2 | Bis-GMA and TEGDMA | Zirconium, silica | 66 |
| Filtek P90 | Silorane based microhybrid composite | 3M/ESPE, St. Paul, MN, USA | A2 | 3,4-Epoxycyclohexylethylcyclopolymethylsiloxane, bis-3,4-poxycyclohexylethylphenylmethylsilane | Silanized quartz, yttrium fluoride | 76 |
Cell Culture Method
After obtaining the approval from the institutional ethical committee, human gingival fibroblasts (HGF) for this study were obtained from the healthy gingiva (without diabetes, hormonal imbalance, and drug induced gingival hyperplasia, devoid of gingivitis and other periodontal problems) of two volunteer patients undergoing oral prophylactic procedures in Manipal College of dental sciences, Mangalore, India. Informed consents were obtained from patients prior to extractions according to institutional ethical committee regulations. Gingival biopsy was taken by giving a local anesthetic and a sulcular incision was made around the tooth and a flap elevated to a limited extent (i.e., the flap was not raised to the mucogingival junction). Remaining gingival tissue tags were removed by thorough curetting of the cervical area of the root surface. Immediately after removal, the tissue was washed twice with Hanks’ salt solution containing with 10% antibiotics (penicillin+streptomycin). Specimens dispersed on glass slides were minced into small pieces and the cut biopsies were plated in 6-well tissue culture plates in Dulbecco’s modified Eagle medium (Sigma, UK) supplemented with 10% fetal calf serum (Biowest Ltd., UK), 2% l-glutamine (Sigma), 100 U/ml penicillin, (Sigma), and 100μg/ml streptomycin (Sigma) and incubated at 37°C in a humidified atmosphere of 5% carbon dioxide in air. Once cells starts to grow [Table/Fig-2a] [17], the adherent cells (fibroblast) were washed with HBSS to remove the large mass of tissues. After 80% confluence, the cells [Table/Fig-2b,3a] were passaged and frozen to use when required.
A) Initial stage of the culture of fibroblast (#) from the gingival biopsy specimen (*) (B) Confluent fibroblast cells after one week of culture

A) Gingival fibroblast primary culture flask (B) Gingival fibroblast seeded for MTT assay in 96-well tissue culture plate

Treatment
After recovering the frozen cells, cells were grown to reach about 80% confluent. Then the cells were trypsinized and counted using Neubauer’s chamber. Ten thousand cells were plated in 96 well plates in triplicate. After attachment of cells, the cells were treated with different doses of the Filtek P90 and Z100 for 24h and 48 h. Then these cells were subjected to MTT assay to assess the proliferation and viability.
MTT Assay
The cells were seeded into a 96 well plate [Table/Fig-3b] at a density of 104 cells/well and incubated for 48 hours to allow them to attach. The attached cells were then treated with various doses of both the composites. DMSO treated cells and untreated cells served as control. Culture was incubated for 24h and 48h. At the end of the incubation period, 20 μ of MTT stock solution {3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide} was added to each well and were incubated for 4 h at 37°C. Then the purple colored formazan crystals were formed due to the reduction activity of the oxidoreductase enzymes in the cells. These crystals were then dissolved in lysis solution (1:24 ratio of HCl: isopropanol) for 1hr at room temperature. The absorbance of the purple coloured solution was measured using ELISA plate Reader at wavelength 570nm [18]. Each experiment was done in triplicates. Readings were analysed using Microsoft Excel program.
Statistical Analysis
The data were presented as mean and SD. The effects of the two composites on the HGF was compared by t-test and to compare the toxicity within the various concentration of composite used and time period one-way ANOVA was used followed by Tukey’s multiple comparison test at significance level of p < 0.05.
Results
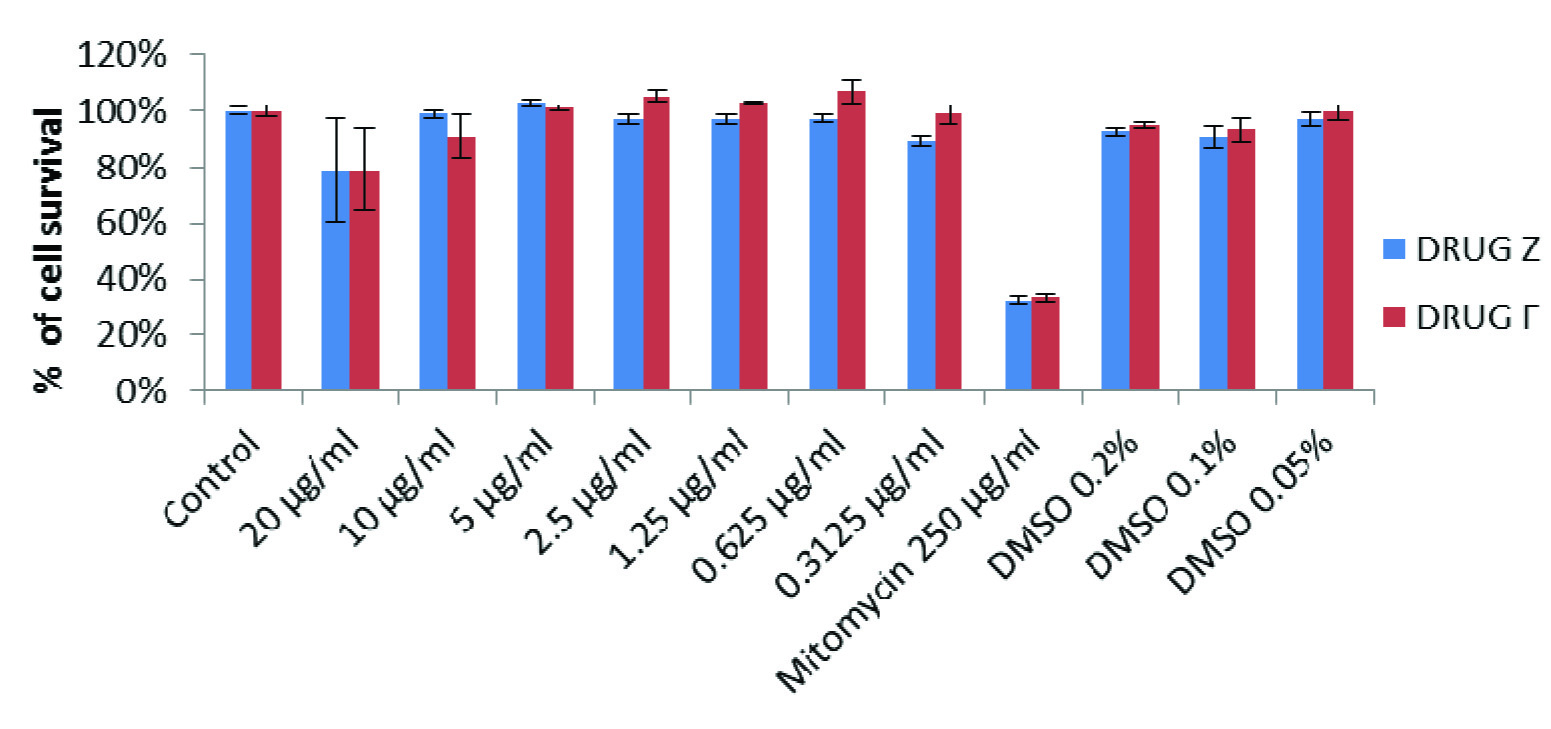
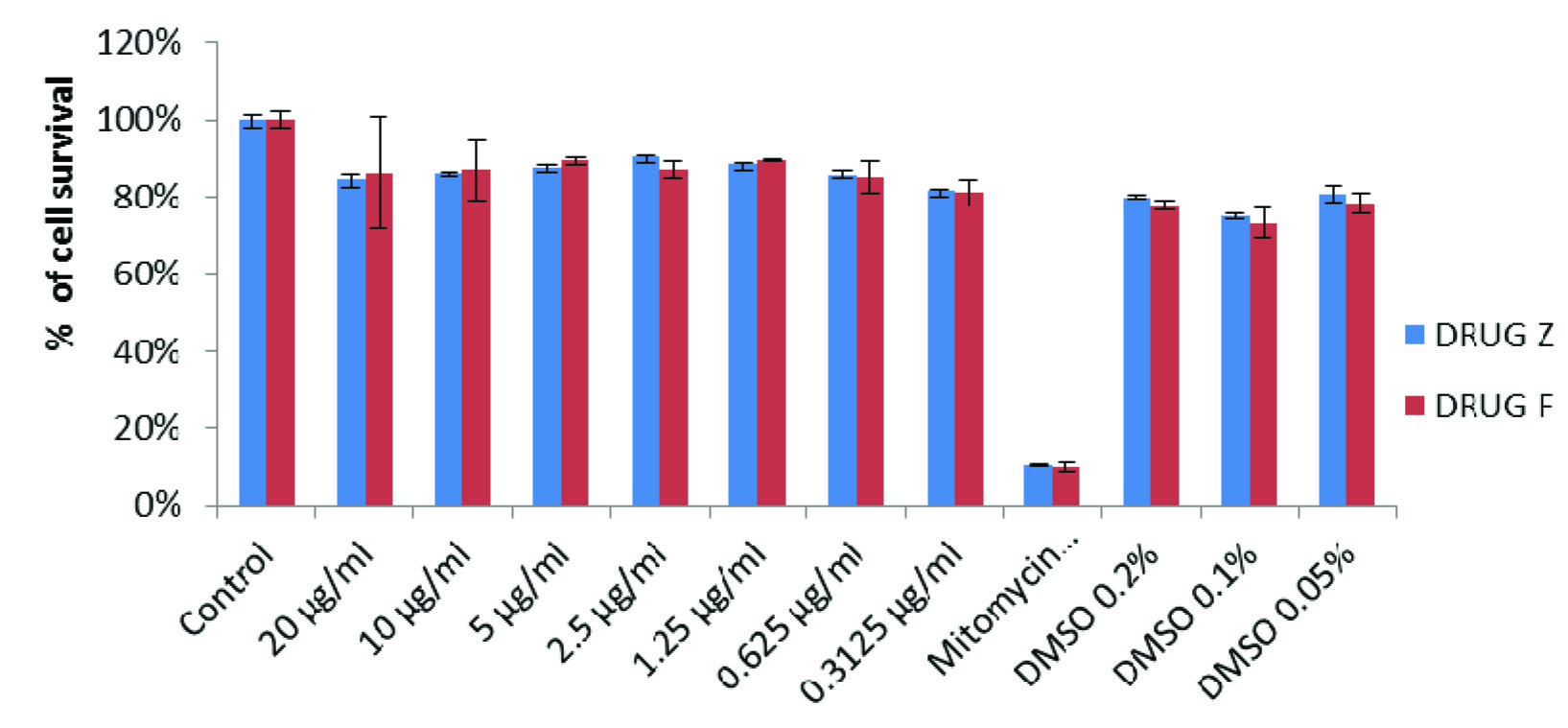
The results of the cytotoxicity assay showed that the percentage of viable cells was very good in the first 24h of treatment and marginally decreased in 48 h of treatment in all groups. However, the proliferation rate was never below 84% in all the groups, at any given concentration.
Result of the MTT assay demonstrated that the survival rate of cells in DMSO (solvent control) alone was about 73% in comparison with untreated group. The percent cell survival rate at different doses of Filtek P90 (drug F) and Z100 (drug Z) was calculated in comparison with the untreated control. Filtek P90 and Z100 treated cells exhibited insignificant decrease (about 15%) in the cell proliferation both in 24h and 48h exposure. Whereas mitomycin (positive control) treated cells (at concentration of 250μg/ml) showed significant decrease (70%) in the cell survival rate compared to all other groups. While comparing the toxicity of Filtek P90 and Z100 treated cells in 24h & 48 h separately [Table/Fig-4,5], it was found that there was no significant difference between these two composites after 24 h of treatment at all concentrations. Treating the cells with 10 μg/ml concentration of Z100, showed significantly higher toxicity (p=0.032) than that of Filtek P90 after 48 h of treatment. However, after 48 h of treatment, the Z100 was found to be little more toxic than the Filtek p90. When compared the rate of cell proliferation 24h and 48h of treatment, the cells treated with 20 μg/ml of Z100 showed significantly (p < 0.05) lower rate of proliferation after 48h. Similarly the cells treated with 20 & 10 μg/ml of Filtek P90 showed significantly (p < 0.05) lower cell growth after 48 h of treatment. Overall no significant alteration in the cell growth was found either in Z100 treated cells or Filtek P90 treated cells.
Comparison proliferation of gingival fibroblasts treated with drug Z and drug F for 24 hours
Comparison of proliferation of gingival fibroblasts treated with drug Z and drug F for 48 hours
Discussion
Though the methacrylate based composites are popularly & widely used, efforts are being made to overcome the clinical deficiencies by recent development and refocusing from the filler content to the matrix resin. There has been dearth of reports in literature on the biocompatibility of siloranes. Present result of nontoxic nature of the materials is in accordance with the observations of others on methacrylates [19,20] and siloranes [21]. However, there are conflicting reports on the cytotoxicity of these materials [21–25].
In the present study, the cytotoxicity observed was mild in both the materials at any given concentrations. The non-cytotoxicity of these composites could be due to the following reasons:
1. Hydrolytic stability of the material. As showed by Palin et al., 2005 [26] that compared to methacrylate based composites (Z250), silorane- based composite exhibits lower solubility, water sorption and diffusion coefficients. These hydrophobic properties diminishes the release of unpolymerized monomers to the oral cavity thus reducing the toxicity [27]
2. Lower levels of residual monomers after polymerization. Monomers released due to lower degree of conversion after incomplete polymerization, can increase the cytotoxic effect of the composite resins [21].
3. Insufficient release of leachable components that produces the cytotoxicity. The low cytotoxicity does not imply absence of leached components. But if, the process of leaching of toxic compounds is slow, it may not reach the lethal dose at any given time to cause the cytotoxicity in the oral cavity. In the present study also, Filtek P90 and Z100 exhibited low level of cytotoxicity as incubation period increased suggesting that these composites have limited leaching of the cytotoxic components into surrounding media [Table/Fig-5]. However, there is a contradicting report which has described a lower degree polymerization of methacrylate-based composites (Filtek Z250) [28] which may release the cytotoxic substances.
Earlier, it has been shown that monomer release from composite resins is complete in 24 h [8,29]. Therefore, most toxic effects from composite resins occur during first 24h. In our present study no significant cytotoxicity was found even after exposing for 48h. However, further evaluation of effect of residual monomers on apoptosis, oxidative stress, delay in cell and molecular mechanism, remains to be elucidated so that the cytotoxicity of resinous materials or monomers can be analysed.
Conclusion
The Filtek P90 and Z100 restorative materials do not cause cytotoxicity in human gingival fibroblasts and are regarded safe when tested in vitro. The new silorane based restorative composite showed comparable cytotoxic characteristics to clinically successful dimethacrylate composites suggesting the non-toxic nature in the oral environment and hence contributing to clinical success of these new restorative materials.
[1]. Kopperud HM, Schmidt M, Kleven IS, Elution of substances from a silorane-based dental compositeEur J Oral Sci 2010 118(1):100-02. [Google Scholar]
[2]. Geurtsen W, Lehmann F, Spahl W, Leyhausen G, Cytotoxicity of 35 dental resin composite monomers/ additives in permanent 3T3 and three human primary fibroblast culturesJ Biomed Mater Res 1998 41:474-80. [Google Scholar]
[3]. Spahl W, Budzikiewicz H, Geurtsen W, Determination of leachable components from four commercial dental composites by gas and liquid chromatography/mass spectrometryJ Dent 1998 26:137-45. [Google Scholar]
[4]. Schweikl H, Spagnuolo G, Schmalz G, Genetic and cellular toxicology of dental resin monomersJ Dent 2006 85(10):870-77. [Google Scholar]
[5]. Hensten-Pettersen A, Skin and mucosal reactions associated with dental materialsEur. J Oral Sci 1998 106:707-12. [Google Scholar]
[6]. Hensten-Pettersen A, Helgeland K, Sensitivity of different human cell line in the biologic evaluation of dental resin-based restorative materialsScand J Dent Res 1981 89:102-07. [Google Scholar]
[7]. van Wyk CW, Olivier A, Maritz JS, Cultured pulp fibroblasts: are they suitable for in vitro cytotoxicity testing?J Oral Pathol Med 2001 30(3):168-77. [Google Scholar]
[8]. Moharamzadeha Keyvan, Noort Richard Van, Brook Ian M, Scutt Andy M, Cytotoxicity of resin monomers on human gingival fibroblasts and HaCaT keratinocytesDent Mater 2007 23:40-44. [Google Scholar]
[9]. Polyzois GL, In vitro evaluation of dental materialsClin Mater 1994 16:21-60. [Google Scholar]
[10]. Theilig C, Tegtmeier Y, Leyhausen G, Geurtsen W, Effects of BisGMA and TEGDMA on proliferation, migration, and tenascin expression of human fibroblasts and keratinocytesJ Biomed Mater Res 2000 53:632-39. [Google Scholar]
[11]. Eick JD, Kostoryz EL, Rozzi SM, Jacobs DW, Oxman JD, Chappelow CC, In vitro biocompatibility of oxirane/polyol dental composites with promising physical propertiesDent Mater 2002 18(5):413-21. [Google Scholar]
[12]. Guggenberger R, Weinmann W, Exploring beyond methacrylatesAm J Dent 2000 13:82D-4D. [Google Scholar]
[13]. Weinmann W, Thalacker C, Guggenberger R, Siloranes in dental compositesDent Mater 2005 21:68-74. [Google Scholar]
[14]. Duarte S Jr, Botta AC, Phark JH, Sadan A Selected mechanical and physical properties and clinical application of a new low-shrinkage composite restorationQuintessence Int 2009 40(8):631-38. [Google Scholar]
[15]. Eick JD, Smith RE, Pinzino CS, Kostoryz EL, Stability of silorane dental monomers in aqueous systemsJ Dent 2006 34:405-10. [Google Scholar]
[16]. Pires-de-Souza Fde C, Garcia Lda F, Roselino Lde M, Naves LZ, Color stability of silorane-based composites submitted to accelerated artificial ageing--an in situ studyJ Dent 2011 39(Suppl 1):e18-24. [Google Scholar]
[17]. Saczko J, Dominiak M, Kulbacka J, Chwilkowska A, Krawczykowska H, A simple and established method of tissue culture of human gingival fibroblasts for gingival ugmentationFolia Histochemica Et Cytobilogica 2008 46(1):117-19. [Google Scholar]
[18]. Nociari MM, Shalev A, Benias P, Russo C, A novel one-step, highly sensitive fluorometric assay to evaluate cell-mediated cytotoxicityJ Immunol Methods 1998 213(2):157-67. [Google Scholar]
[19]. Al-Hiyasat AS, Darmani H, Milhem MM, Cytotoxicity evaluation of dental resin composites and their flowable derivativesClin Oral Investig 2005 9(1):21-25. [Google Scholar]
[20]. Chen MH, Chen CR, Hsu SH, Sun SP, Su WF, Low shrinkage light curable nanocomposite for dental restorative materialDent Mater 2006 22(2):138-45. [Google Scholar]
[21]. Brackett MG, Bouillaguet S, Lockwood PE, Rotenberg S, Lewis JB, Messer RL, Wataha JC, In vitro cytotoxicity of dental composites based on new and traditional polymerization chemistriesJ Biomed Mater Res B Appl Biomater 2007 81(2):397-402. [Google Scholar]
[22]. Tunçel A, Ozdemir AK, Sümer Z, Hürmüzlü F, Polat Z, Cytotoxicity evaluation of two different composites with/without fibers and one nanohybrid compositeDent Mater J 2006 25(2):267-71. [Google Scholar]
[23]. Schweikl H, Hiller KA, Bolay C, Kreissl M, Kreismann W, Nusser A, Cytotoxic and mutagenic effects of dental composite materialsBiomaterials 2005 26(14):1713-19. [Google Scholar]
[24]. Franz A, König F, Anglmayer M, Rausch-Fan X, Gille G, Rausch WD, Cytotoxic effects of packable and nonpackable dental compositesDent Mater 2003 19(5):382-92. [Google Scholar]
[25]. Schedle A, Franz A, Rausch-Fan X, Spittler A, Lucas T, Samorapoompichit P, Cytotoxic effects of dental composites, adhesive substances, compomers and cementsDent Mater 1998 14(6):429-40. [Google Scholar]
[26]. Palin WM, Fleming GJ, Burke FJ, Marquis PM, Randall RC, The influence of short and medium-term water immersion on the hydrolytic stability of novel low-shrink dental compositesDent Mater 2005 21:852-63. [Google Scholar]
[27]. Eick JD, Kotha SP, Chappelow CC, Kilway KV, Giese GJ, Glaros AG, Properties of silorane-based dental resins and composites containing a stress-reducing monomerDent Mater 2007 23:1011-17. [Google Scholar]
[28]. Marchesi G, Breschi L, Antoniolli F, Di Lenarda R, Ferracane J, Cadenaro M, Contraction stress of low-shrinkage composite materials assessed with different testing systemsDent Mater 2010 26:947-53. [Google Scholar]
[29]. Ferracane JL, Condon JR, Rate of elution of leachable components from compositeDent Mater 1990 6:282-87. [Google Scholar]