One of the common diseases affecting collagen turnover and degradation is Oral Submucous Fibrosis (OSMF). OSMF is a chronic, progressive, scarring, potentially malignant disorder of the oral mucosa characterized by a juxta epithelial inflammatory reaction followed by a fibro elastic change in the lamina propria and associated epithelial atrophy [1]. The current understanding of OSMF is that it is a ‘collagen metabolic disorder’. Collagen being a triple helix of amino acids is sensitive to changes in the amino acid profiling.
Studies on amino acids are making a major contribution in understanding of disease. Amino acid therapies have been used successfully to prevent aging, heart disease, enhance memory, relieve stress, arthritis [2]. The complexity of metabolic derangement of protein in patients may be reflected by alterations in plasma amino acids profile [3]. Few studies exist on use of metabolic profiling of amino acids to examine the underlying physiological or disease state [4]. High performance liquid chromatography (HPLC) is an efficient way of separating the amino acids and measuring them from minute quantities of plasma [5].
An autofluorescence study has shown significant difference between the spectrum of collagen in OSMF and normal mucosa [6]. Studies have shown that amino acids and their derivatives can provide some useful markers that can reflect the protein metabolism status in various conditions, as plasma levels are the preferred way to assess long term adequacy and dynamics of amino acid utilization. ‘Hydroxyproline’ appears to be the hallmark for celiac disease and other malabsorption states [7]. Comparison of patients with head and neck cancers with controls have demonstrated significant differences (increased lysine and decreased arginine levels) [8–11]. The plasma amino acid profile in patients with potentially malignant disorders and collagen metabolic disorders like OSMF are less well defined. Considering the above facts, the present study was attempted to evaluate the amino acid profile; to correlate it with the etiopathogenesis and metabolic derangement and to assess its feasibility as a biological marker in OSMF by the HPLC method.
Materials and Methods
The study group comprised of 13 newly diagnosed cases of OSMF and control group of 13 normal individuals without habits and was conducted over a six month period. The study group comprised of 13 subjects, of whom 4 were classified as mild, 5 as moderate and 4 as severe cases of OSMF based on the classification by Khanna JN and Andrade NN (1995) [12]. The patients were accordingly divided into the following groups: GROUP I – MILD; GROUP II – MODERATE; GROUP III – SEVERE; GROUP IV – CONTROL.
The patients were selected from the outpatient department of Saveetha dental college and Hospital [Table/Fig-1]. The protocol was approved by the Ethics Committee and Institutional Review Board at Saveetha University, Chennai, India with the ethical committee approval {(ihep no.) MDS 35/SU 64/06}. All experiments on human subjects were conducted in accordance with the Declaration of Helsinki (http://www.wma.net) and that all procedures were carried out with the adequate understanding and written consent of the subjects.
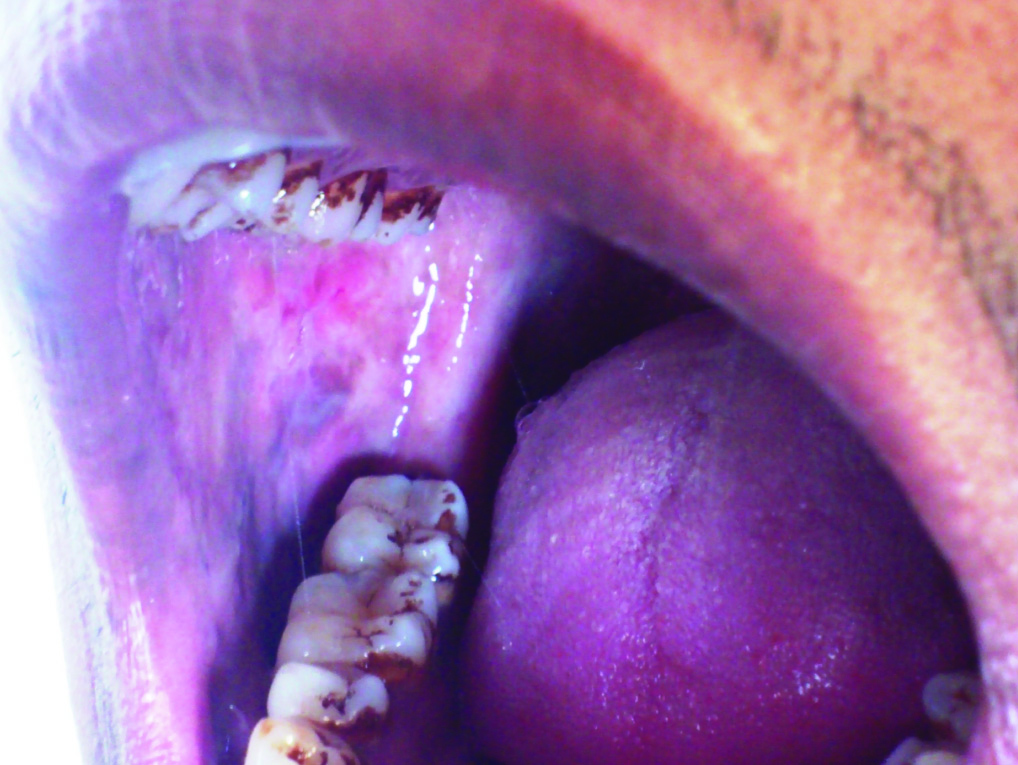
Blotchy marble like pallor of buccal mucosa in a patient of OSMF
Diagnostic criteria used for selection of cases included:
HISTORY - History of betel nut, tobacco, pan, gutka chewing etc.
SYMPTOMS – a) progressive inability to open the mouth. b) Progressive inability to protrude the tongue c) burning sensation of oral mucosa [Table/Fig-2&3].
SIGNS – pale blanched mucosa, firm in consistency, associated with vertical, palpable fibrotic bands in buccal mucosa.
Measurement of interincisal distance in a patient of OSMF
Measurement of extent of tongue protrusion in a patient of OSMF

The grading for fibrotic bands was as follows:
Normal – mucosa is pink, soft, elastic; Mild – mucosa is pale, yields but not fully, slight blanching of mucosa; Moderate – mucosa is firm, but yields on pressure; Severe – mucosa is firm to hard, does not yield on finger pressure.
Inclusion Criteria
Patients diagnosed with OSMF, who were not suffering from any other systemic diseases like diabetes, hypertension or any autoimmune diseases.
Exclusion Criteria
Patients diagnosed with OSMF along with other associated systemic ailments like diabetes, hypertension other associated precancerous lesions or conditions and any other auto immune disease.
Blood Sample Collection
Fasting blood samples were collected from ante cubital vein, into acid citrate dextrose containing vacuettes. The Samples were then centrifuged at 2500 rpm for 10 min to separate the plasma, stored at 4°C and sent for HPLC analysis of Amino Acids to Department of Biochemistry, Sankara Nethralaya, Chennai, India.
Procedure
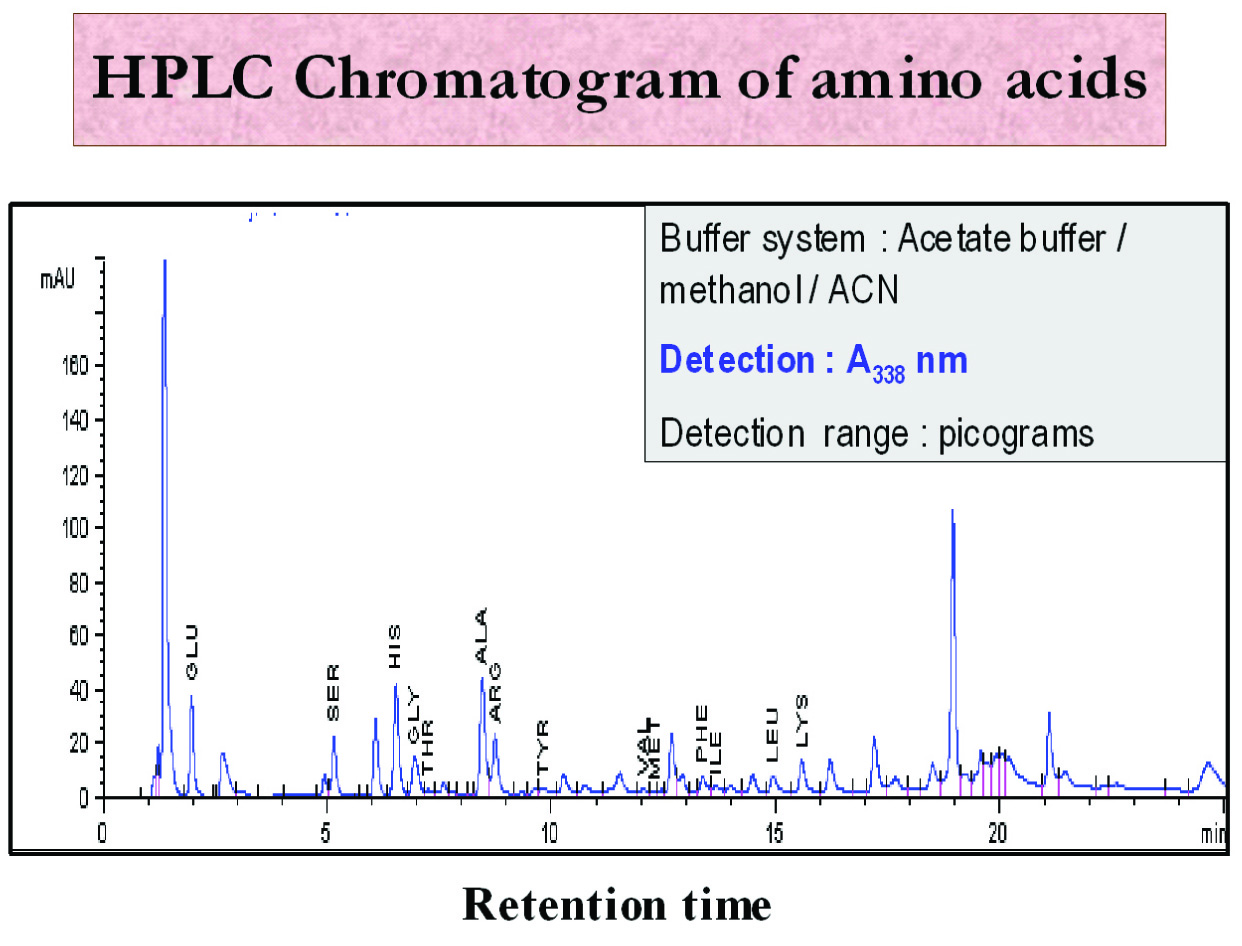
The Agilent 1100 HP from Agilent Technologies HPLC system was used in the present study. (Hewlett Packard, Strasse-8, Waldbronn, Germany) [Table/Fig-4]. Standard (10 μl) was mixed in 60 μl of borate buffer and 10 μl of reagent in a dilution vial and final mixing was carried out by a high shear, fast intensive mixer (Cyclomix, Hosokawa Micron Ltd, Runcorn, Cheshire, UK). From this mixture, 50 μl was injected into the HPLC system using Hamilton syringes. Each standard was individually run on the gradient noted in the LC (Liquid chromatography) parameters program (Reaction temperature: 40°C, pressure: 100 bars, column size: 3x150 mm, column bed volume: 0.64 ml, flow rate: 0.5 ml/min, detection wave length: 338 nm and injection: 50 μl). Chromatograms were obtained [Table/Fig-5]. Two consecutive runs with same retention times were taken, and the means were used for plotting graphs in the calibration table. This procedure was termed as calibration, and the obtained curve was referred to as the calibration curve. From the chromatogram, the area of the peaks were recorded, calibrated along with the standards and used for calculations. Results were expressed in terms of μmol/ml.
HPLC system control and data acquisition

HPLC chromatogram of amino acids in OSMF
Statistical Analysis
The significance of the results obtained from the control and study groups was statistically analyzed by Kruscal Wallis test and Mann Whitney u test. p-values of <0.05 were considered to be statistically significant. The statistical analysis was carried out using SPSS software. Mann- whitney U-test is used to test the differences between two independent groups when the dependent variable is either ordinal or interval/ratio, but not normally distributed.
Results
The results of our study showed that assay levels of histidine, threonine, arginine, tyrosine, isoleucine and leucine were decreased in all the groups of OSMF. Levels of alanine, methionine was found to be increased in all groups of OSMF. Levels of serine and glycine were decreased in mild and severe group, increased in moderate group whereas the levels of valine, phenylalanine and lysine were found to be increased in mild group and decreased in moderate and severe group of OSMF [Table/Fig-6].
Mean value of various amino acids
| AMINO ACIDS | GROUP I (Mild) | GROUP II (Moderate) | GROUP III (Severe) | GROUP IV (Control) |
|---|
| Serine (S) | 0.15 mg/dl Decreased | 1.48 mg/dl increased | 0.78 mg/dl decreased | 0.85 mg/dl |
| Histidine (H) | 5.47 mg/dl Decreased | 6.90 mg/dl decreased | 5.63 mg/dl decreased | 10.5 mg/dl |
| Glycine (G) | 1.82 mg/dl Decreased | 2.44 mg/dl increased | 1.27 mg/dl decreased | 2.00 mg/dl |
| Threonine (T) | 2.04mg/dl Decreased | 1.92 mg/dl decreased | 1.26mg/dl decreased | 2.35mg/dl |
| Alanine (A) | 5.04mg/dl Increased | 3.75 mg/dl increased | 3.57mg/dl increased | 3.50mg/dl |
| Arginine (Ar) | 4.09mg/dl Decreased | 3.83 mg/dl decreased | 4.22mg/dl decreased | 10.50mg/dl |
| Tyrosine (Ty) | 1.39mg/dl Decreased | 1.34mg/dl decreased | 1.09mg/dl decreased | 1.5mg/dl |
| Valine (V) | 1.94mg/dl Increased | 0.91mg/dl decreased | 1.15mg/dl decreased | 1.91mg/dl |
| Methonine (M) | 3.95mg/dl Increased | 4.94mg/dl increased | 3.81mg/dl increased | 2.5mg/dl |
| Phenyalanine (P) | 1.96mg/dl Increased | 0.63mg/dl decreased | 0.61mg/dl decreased | 1.50mg/dl |
| Lysine (Ly) | 2.70mg/dl Increased | 1.48mg/dl decreased | 1.16mg/dl decreased | 2.50mg/dl |
| Isoleucine (I) | 1.31mg/dl Decreased | 0.63mg/dl decreased | 0.61mg/dl decreased | 1.7mg/dl |
| Leucine (L) | 1.54mg/dl Decreased | 0.75mg/dl decreased | 1.78mg/dl decreased | 3.50mg/dl |
The statistically significant decreased assay levels observed in Arginine, Leucine in all the study groups of OSMF (I,II and III); the increased assay level of lysine in group I and decreased assay level in group II and group III when compared to the control group shows promise as disease markers [Table/Fig-7].
Statistical significance of the correlation between the study groups and control group
| AMINO ACIDS | Group I & II | Group I&III | Group I & IV | Group II & III | Group II & IV | Group III&IV |
|---|
| Serine (S) | 0.014 | 0.043 | 0.003 | 0.221 | 0.520 | 0.493 |
| Histidine (H) | 0.624 | 1.00 | 0.013 | 0.624 | 0.054 | 0.020 |
| Glycine (G) | 0.325 | 0.149 | 0.212 | 0.027 | 0.006 | 0.427 |
| Threonine (T) | 1.00 | 0.149 | 1.00 | 0.462 | 0.054 | 0.140 |
| Alanine (A) | 0.221 | 0.149 | 0.135 | 0.624 | 0.517 | 0.135 |
| Arginine (Ar) | 0.462 | 0.248 | 0.003 | 0.327 | 0.001 | 0.003 |
| Tyrosine (Ty) | 0.806 | 0.386 | 1.00 | 0.621 | 0.518 | 0.137 |
| Valine (V) | 0219 | 0.386 | 1.00 | 0.325 | 0.001 | 0.137 |
| Methionine (M) | 0.624 | 0.773 | 0.137 | 0.624 | 0.001 | 0.137 |
| Phenylalanine (P) | 0.142 | 0.248 | 0.139 | 1.00 | 0.001 | 0.003 |
| Lysine (Ly) | 0.014 | 0.021 | 0.303 | 0.327 | 0.001 | 0.003 |
| Isoleucine (I) | 0.221 | 0.885 | 0.136 | 0.327 | 0.001 | 1.00 |
| Leucine (L) | 0.327 | 1.00 | 0.003 | 0.268 | 0.001 | 0.003 |
The decreased assay level of serine in group I in comparison with all the other study groups yielded statistical significance. The decreased assay levels of histidine of group I and III in comparison with the control group is statistically significant. The increased assay levels of Glycine in group II in comparison with the control as well with the severe group are statistically significant.
The assay levels of threonine, alanine and tyrosine did not yield any significant results. The decreased assay levels of valine, Isoleucine and the increased assay level of methionine and glycine observed in group II yielded significant results in correlation with the control group. The decreased assay level seen in phenylalanine in group II and III in correlation with group IV is statistically significant.
Discussion
Studies have shown that amino acids and their derivatives can be useful markers that reflect the protein metabolism. Low levels measured among essential and some semi essential amino acids reflect dietary and uptake problem. Elevation in amino acids levels can serve as “Disease Marker”. Hydroxyproline appears to be a hallmark of celiac disease and other malabsorption states. Alterations in plasma levels of Aspartate, Glutamate, Glycine and Tyrosine have been suggested as neurochemical marker for epilepsy [5].
Metabolic aspects in head and neck carcinogenesis have been studied less extensively. Metabolic alterations often not specific, are frequently associated with carcinoma. Laviano A [13], Cascino A, Muscaritoli M, et al., [14] stated that tumour growth is associated with a number of metabolic abnormalities. They have suggested that Tryptophan can be used as a marker of neoplastic disease.
Keeping these facts in mind, the present study was carried out to evaluate whether amino acid profile in patients with precancerous condition such as OSMF, can show alterations. This study, to the best of our knowledge, is the first attempt to evaluate the role of amino acid profile in OSMF.
Out of the known 20 essential and non essential amino acids, 13 amino acids were selected, namely Serine, Histidine, Glycine, Threonine, Alanine, Tyrosine, Valine, Methonine, Phenyalanine, Lysine, Arginine, Isoleucine, Leucine. These 13 amino acids were selected on the basis that these follow the same protocol, as on analysis of the samples these are detected at the same peak value (380nm).The remaining four which are sulphur containing amino acids were excluded as these have different detection peak values hence difficult to differentiate for analysis. Collagen related amino acids namely proline, hydroxyproline, and hydroxylysine require fluorescence detector, and due to non availability of the facility these were also excluded [15].
OSMF is a “collagen metabolic disorder” where there is an overall increased collagen production and decreased collagen degradation leading to fibrosis [3]. Till date, the studies in OSMF have mainly concentrated on hydroxyproline, proline, glycine and hydroxylysine.
The results of our study showed an alteration in plasma amino acid level in OSMF patients as compared to control. The assay levels observed in Arginine, Leucine in and lysine implies that these amino acids in particular shows promise as disease markers [Table/Fig-7].
The reduction in the assay levels of histidine, threonine, arginine, tyrosine, isoleucine and leucine in all groups of OSMF owes to the fact that these amino acids aids in healing and repair of muscle tissue, skin and bones; threonine specifically helps in the formation of collagen and elastin; tyrosine has a role in overall metabolism. Therefore, the reduction directly relates to their being used up as part of the pathophysiology of the disease process contributing to an overall anabolic state. The reduction in arginine also correlates well with the studies done by Yvonne LJ Visser et al., [16] who had reported that arginine metabolism is disturbed in cancer patients. They had found plasma arginine levels to be low in patients with cancer, attributing it to the various degrees of metabolic derangements in malignant condition, even without weight loss. This suggests that decreased arginine availability can be a specific feature of presence of cancer. This gains importance in our study also since correlation values of arginine, lysine and leucine in all the study groups with the control group yielded statistical significance.
Levels of serine and glycine were decreased in mild and severe group, increased in moderate group. Serine is required for muscle growth and for the maintenance of a healthy immune system. OSMF being an overall anabolic process and in lieu of it being considered to be an autoimmune process is responsible for the reduction in the assay levels in the mild and severe group. Glycine is the major building block of collagen and has an effect of retardation of muscle degeneration, also contributes to healing and repair and thus is expected to have reduced levels. There appears to be a discrepancy in the moderate stage where the levels of both serine and glycine are increased [17]. This could probably be due to a reduction in the collagen formation or a stable state being reached temporarily in the moderate stage of the disease. The decreased assay level of serine in group I in comparison with all the other study groups yielded statistical significance. Group II showed decreased assay levels of glycine yielding statistically significant results on correlation with group III and IV.
The value for Valine, Phenylalanine and Lysine was increased in the mild group but showed a decrease in the moderate group. The values showed a further decrease in the severe group. Thus the profile of these amino acids revealed a progressive decrease with severity of disease.
Cross linking of lysine to make proper collagen is dependent upon the enzyme lysyl oxidase which requires copper. OSMF is a premalignant condition associated with areca nut. Presence of a high concentration of copper in AN (areca nut) may up regulate the lysyl oxidase activity, leading to excessive cross linking and deposition of collagen (JH Jeng, MC Chang et al., Trivedy et al.,) [18,19]. Thus, the level of lysine correlates well with the disease process. The increased assay level of lysine in group I and decreased assay level in group II and III when compared to the control group yielded statistical significance.
L – phenylalanine is used biochemically to form protein. Valine is a branched chain amino acid which promotes protein synthesis, suppresses protein catabolism and serves as substrate for gluconeogenesis. The three amino acids show a progressive reduction in their levels with increasing severity of the disease. This may be attributed to their increased usage in the cross linking and collagen synthesis. The decreased assay levels of valine, and the increased assay level of methionine observed in group II yielded significant results in correlation with the control group. The decreased assay level seen in phenylalanine in group II and III in correlation with group IV is statistically significant. These amino acids could serve as candidate research points as biological markers for OSMF.
Conclusion
OSMF, being a precancerous condition, with the progression of severity has an increased potential for malignant transformation. We have found few amino acids which can be used as biological markers for the severity of the disease. However, further studies are needed to elucidate the potential of these profiles in the pathogenesis of OSMF and its implications in the malignant transformation potential of such condition.