Botryoid Odontogenic Cyst: A Diagnostic Chaos
Anuradha A1, Urmila U2, Vijay Srinivas G3, Sabitha Deviramisetty4, Puneeth HK5
1 Professor, Department of Oral and Maxillofacial Pathology, St Joseph Dental College, Eluru, Andhra Pradesh, India.
2 Post-Graduate Student, Department of Oral and Maxillofacial Pathology, St Joseph Dental College, Eluru, Andhra Pradesh, India.
3 Professor and Head of Department, Department of Oral and Maxillofacial Pathology, St Joseph Dental College, Eluru, Andhra Pradesh, India.
4 Reader, Department of Oral and Maxillofacial Pathology, St Joseph Dental College, Eluru, Andhra Pradesh, India.
5 Senior Lecturer, Department of Oral and Maxillofacial Pathology, St Joseph Dental College, Eluru, Andhra Pradesh, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Anuradha. A, St Joseph Dental College, Eluru-534004, Andhra Pradesh, India. Phone : 9866867177, E-mail : anuradhaundavalli@yahoo.com
Botryoid Odontogenic cyst (BOC) originally described by Weathers and Waldron (1973) is a variant of a lateral periodontal cyst characterized by macroscopic and microscopic multilocular growth pattern. We report a case of BOC in a 21-year-old male patient. Orthopantamogram revealed a multilocular radiolucency extending from 43 to 47. The histological examination of incisional biopsy revealed a thin 2-4 layered non keratinised epithelium without rete ridges resembling a reduced enamel epithelium with few localised plaque like thickenings and occasional mural bulges. These features were suggestive of BOC. The excisional biopsy revealed histological features similar to those of incisional biopsy except for the presence of 5-6 epithelial follicles with outer columnar cells and inner stellate reticulum like cells. CD56 and calretinin immunohistochemical staining (IHC) was done. This paper highlights the unusual appearance of follicles in BOC with differential diagnosis and IHC staining characteristics.
Ameloblastoma, Botryoid odontogenic cyst (BOC), CD56, Calretinin lateral periodontal Cyst (LPC), Odontogenic keratocyst (OKC)
Case Report
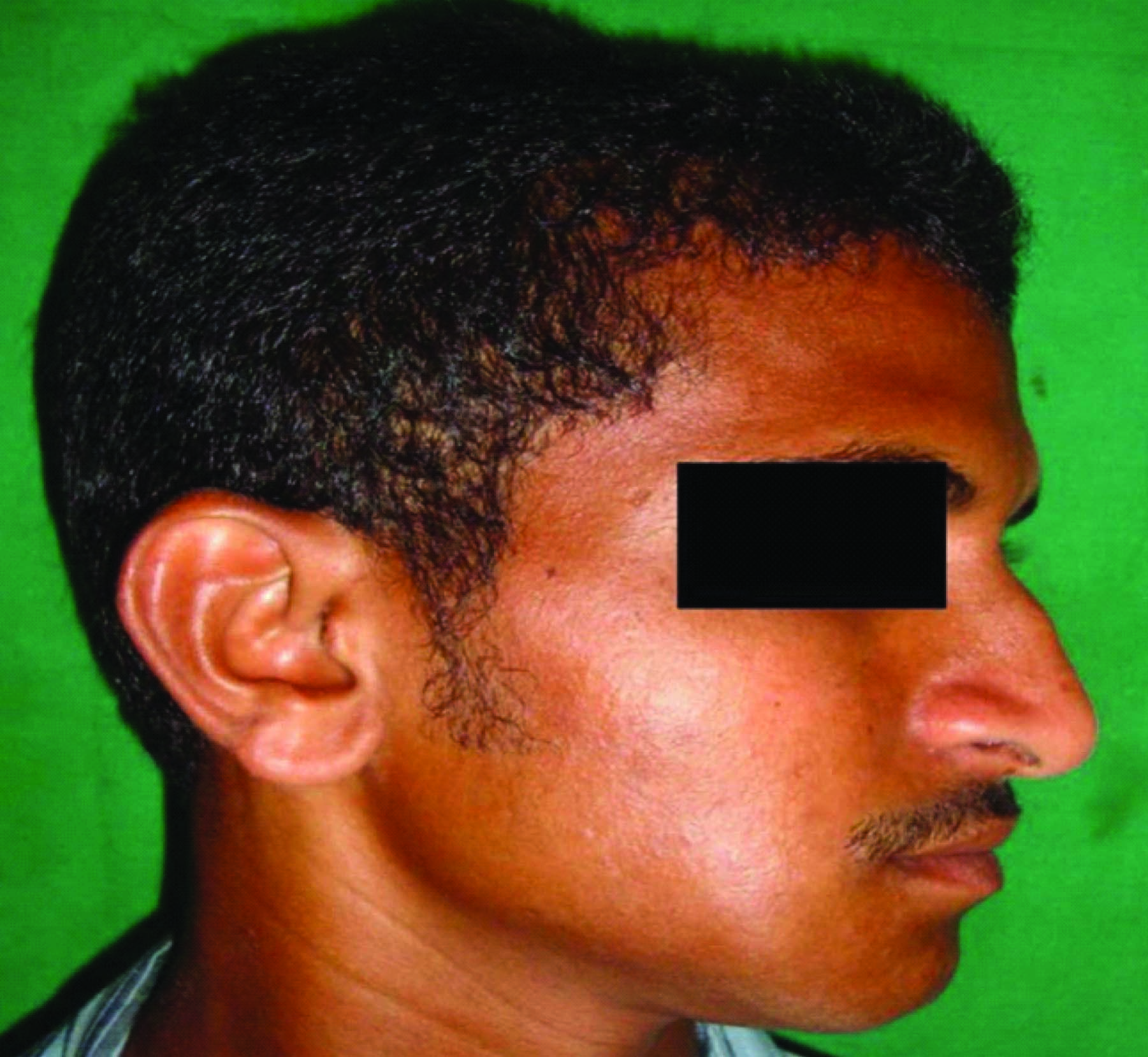
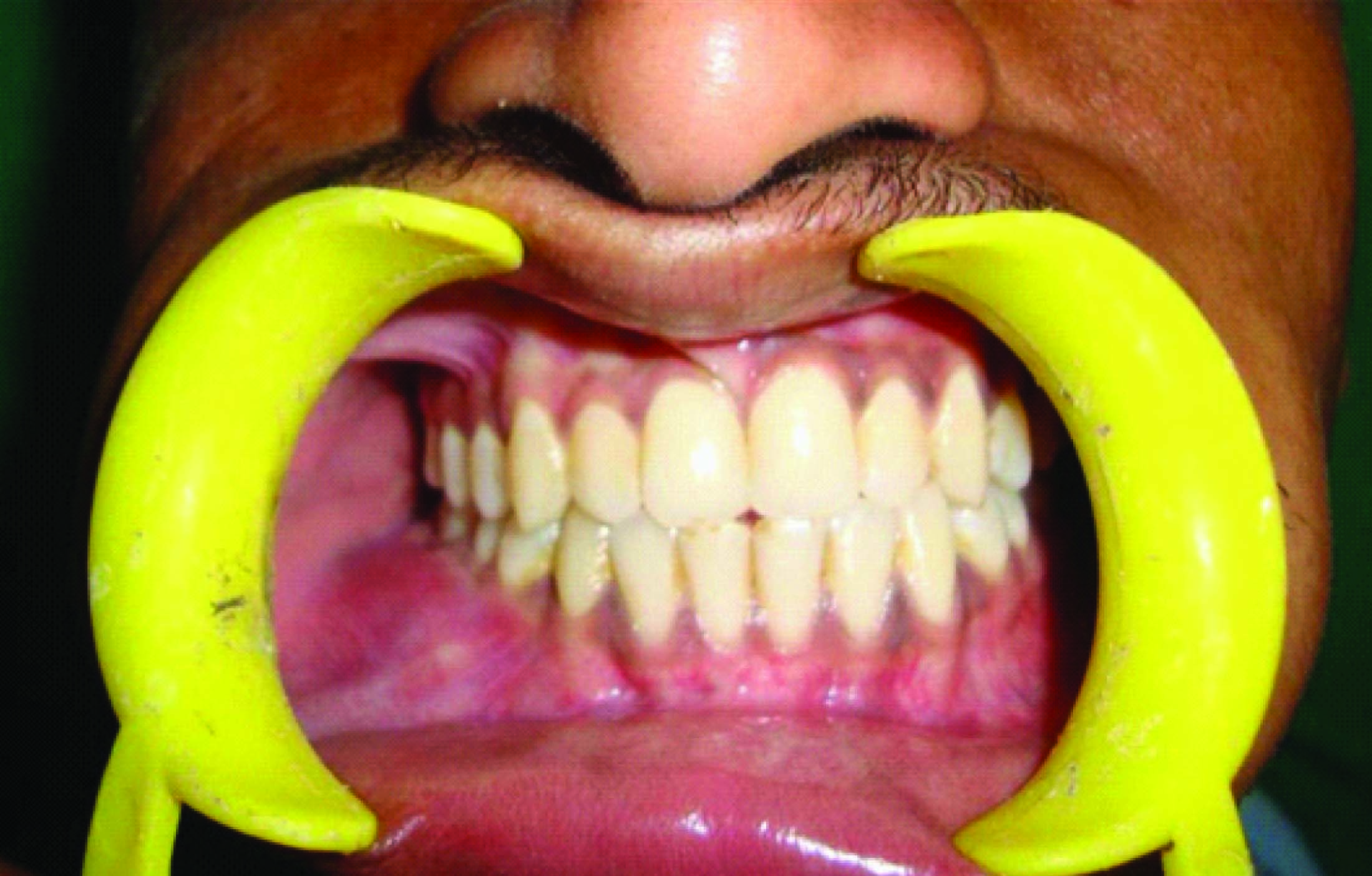
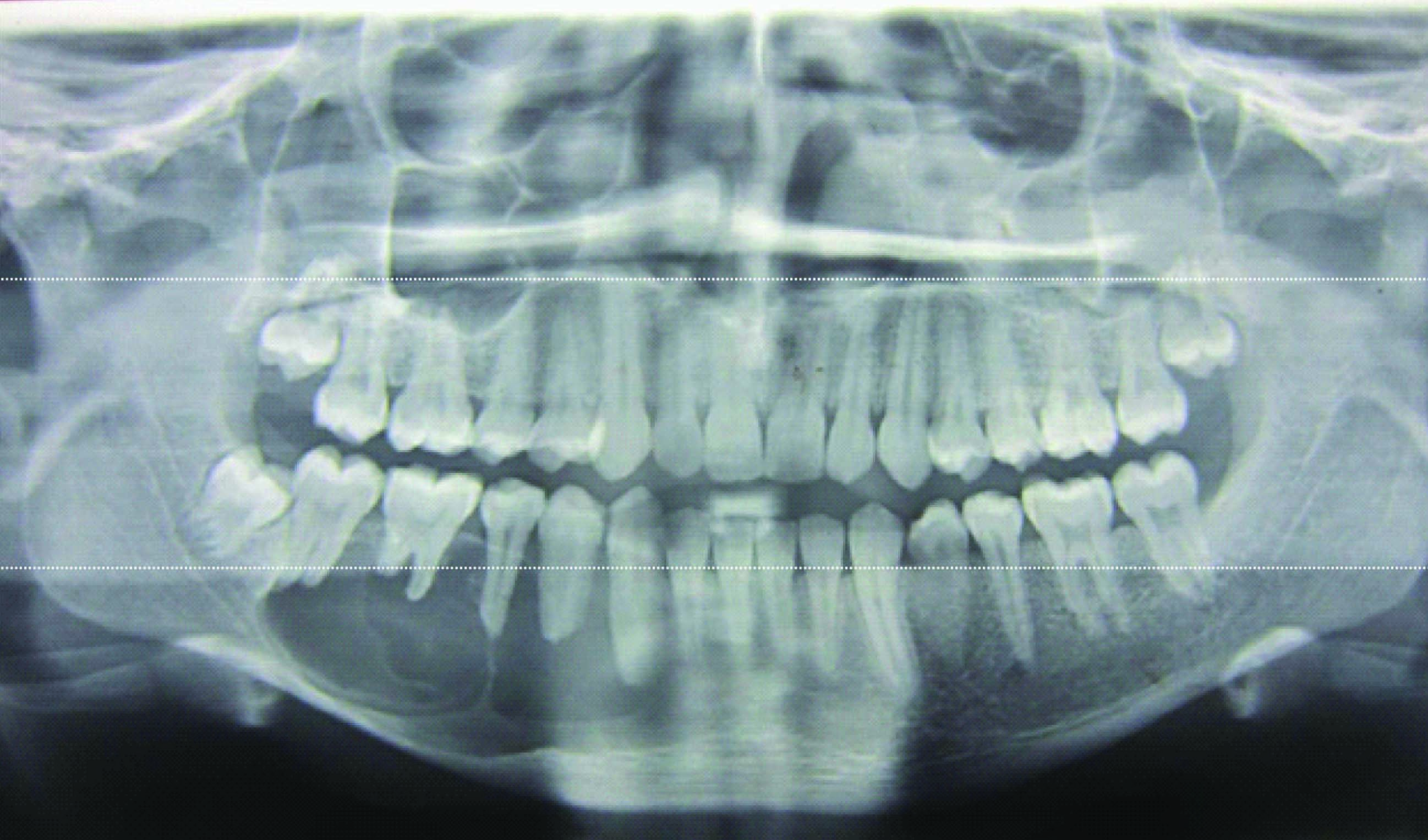
A 21-year-old male patient presented to outpatient department with a history of swelling associated with dull aching continuous pain in the lower right back tooth region since 20 d. Initially the swelling was small which gradually increased to the present size. Clinical examination revealed a mild diffuse extra oral swelling measuring approximately 3x2cm extending anteriorly to the right corner of the lip, posteriorly upto the external oblique ridge, inferiorly 0.5mm above inferior border 9 of mandible and superiorly along the occlusal plane coinciding with the line joining the corner of lip to tragus of ear [Table/Fig-1]. Intraorally the swelling was diffuse extending from 43 to 47 within the intact mucosa obliterating lower right vestibular area [Table/Fig-2]. Palpation revealed firm to hard swelling, expansion of buccal and lingual cortical plates with grade II mobility in relation to 43, 44, 45 and 46. Orthopantamogram and Occlusal radiograph showed a well-delineated multilocular radiolucency extending from mesial aspect of 43 to distal aspect of 47 [Table/Fig-3&4]. The computed tomography scan (CT) and 3D CT revealed perforation of buccal and lingual cortical plates in relation to 46 and 47 [Table/Fig-4&5]. Blood investigations were within normal limits. On aspiration amber coloured fluid mixed with blood was obtained.
Diffuse right extraoral swelling
Intra oral swelling obliterating the vestibule and extending from 43 to 47 region
Orthopantamogram revealing a well-delineated multilocular radiolucency extending from mesial aspect of 43 to distal aspect of 47
Occlusal radiograph showing multilocular radiolucency with perforation of buccal cortical plates in relation to 44 and 46 and the computed tomography scan(CT scan) reveals perforation of buccal and lingual cortical plates in relation to 46 and 47

The 3D-computed tomography scan(CT scan) reveals perforation of buccal and lingual cortical plates in relation to 46 and 47

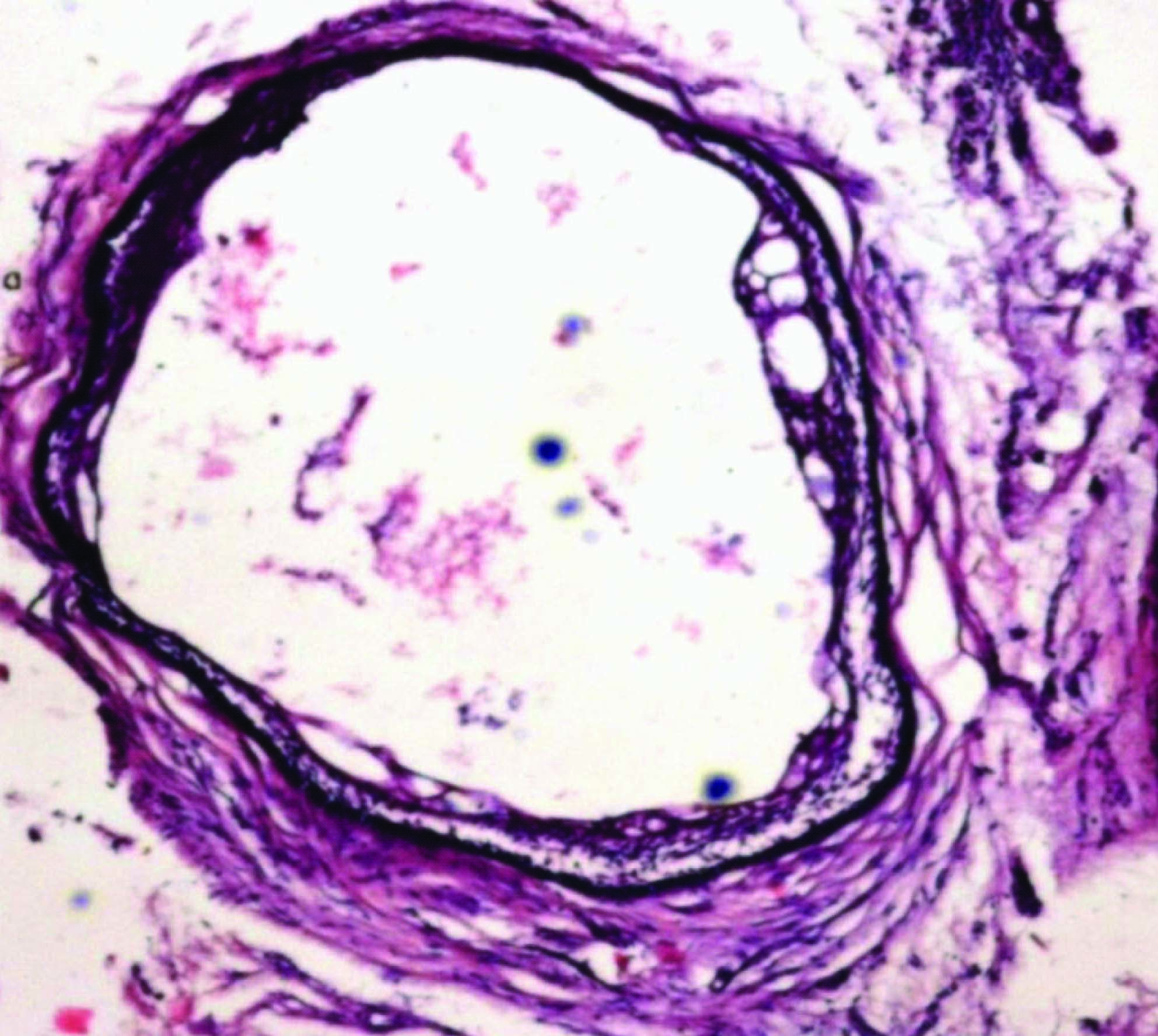
Based on the clinical and radiological features a differential diagnosis of ameloblastoma or odontogenic keratocyst (OKC) was given. An Incisional biopsy was done in 43, 44 region under local anesthesia (LA). The gross specimen was about 1 cm in size, brown in color and soft in consistency. The histopathological examination revealed a thin cystic lining which was 2-3 layered nonkeratinized stratified squamous resembling the reduced enamel epithelium [Table/Fig-6]. Localised epithelial plaques and mural down growths were evident in some areas. The basal cells of the plaque were cuboidal and vacuolated. The superficial cells were fusiform with few areas showing intercellular edema. Multiple small cystic spaces lined by similar cystic epithelium were seen in the fibrous wall along with few epithelial rests and variable amount of chronic inflammatory infiltrate [Table/Fig-7]. Based on these features it was diagnosed as botryoid odontogenic cyst (BOC).
Excisional biopsy was done under local anesthesia. The gross specimen consisted of extracted 43, 44, 45, 46 along with multiple soft tissue pieces which were white in colour, irregular in shape and soft in consistency. Histopathological picture was similar to incisional biopsy except for the presence of 5-6 epithelial follicles showing outer columnar cells and inner stellate reticulum like cells. The whole excisional biopsy specimen was processed and sections were made. None of these showed similar follicles. Immunohistochemical staining using CD56 and Calretinin was done. Cystic epithelial lining showed negativity and the cytoplasm of peripheral columnar cells of the follicles showed faint positivity for CD56 along with the connective tissue stroma [Table/Fig-8]. Calretinin expression was completely negative. Neither the cystic lining nor the epithelial rests or follicles showed positivity [Table/Fig-9]. But some pseudo staining was seen in the connective tissue stroma. Based on these features a confirmatory diagnosis of BOC was given. Lip split incision was placed and the pathological site was exposed. Then surgical enucleation was done along with chemical cauterization using carnoy’s solution. The present case was followed up for every week for two months and later on monthly visit for a period of three years were instituted [Table/Fig-10–12].
Layered nonkeratinized stratified squamous cystic lining resembling the reduced enamel epithelium

Multiple cystic spaces lined by thin nonkeratinized stratified squamous epithelium
CD56 positivity in the peripheral columnar cells of the follicles and the connective tissue stroma and CD56 negativity in the epithelial lining
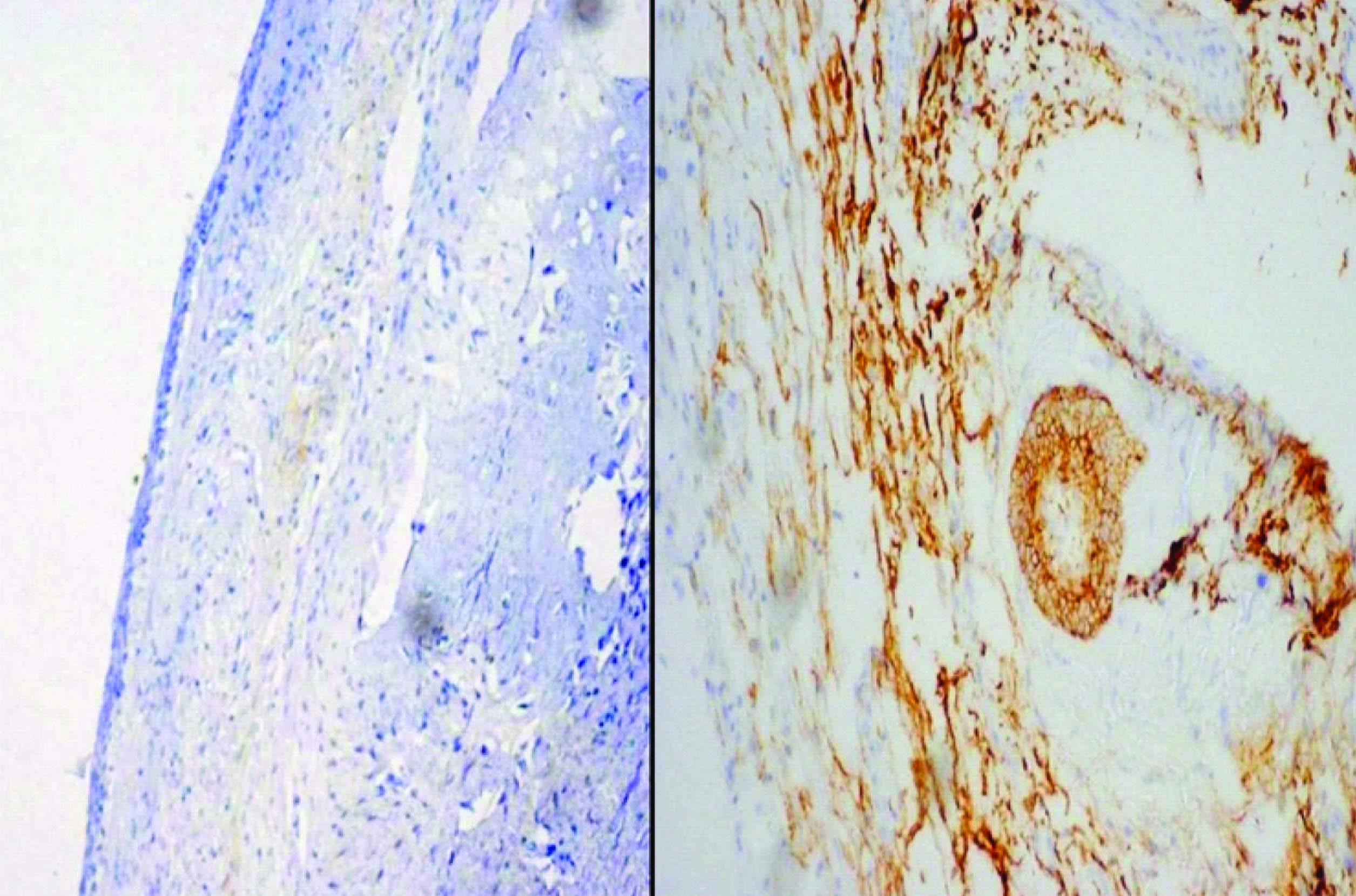
Absence of calretinin expression both in the lining epithelium and the follicles

Postoperative facial picture
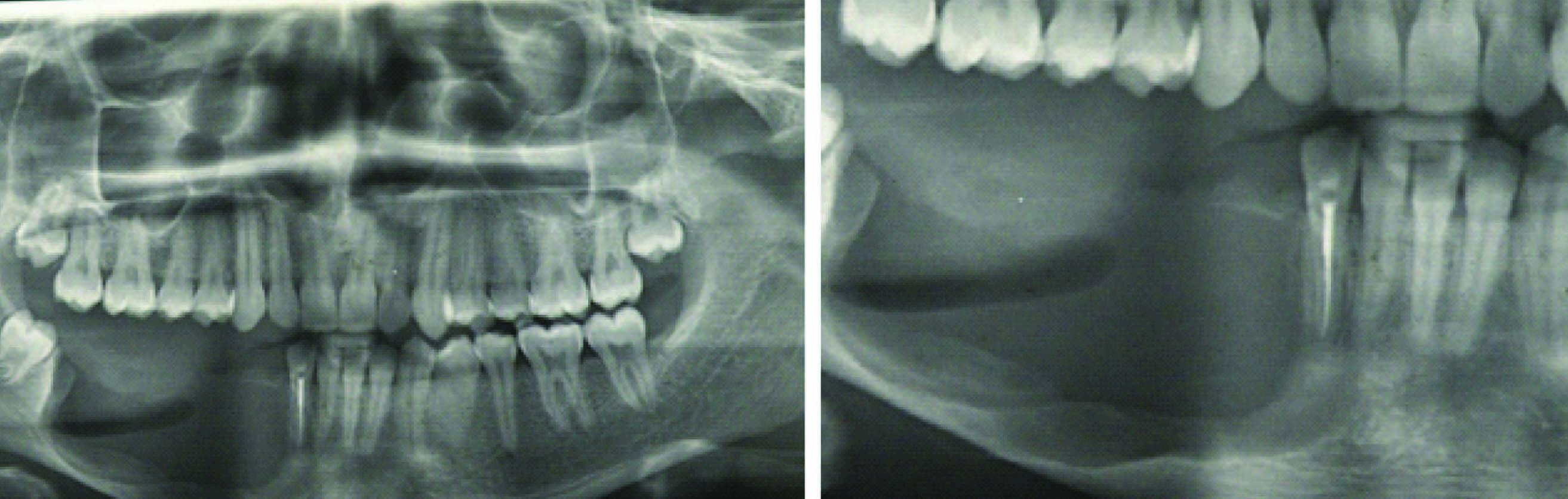
Postoperative orthopantomograph
Discussion
BOC is a multilocular variant of LPC resembling bunch of grapes which is apparently uncommon. The age of occurrence ranges from 23-85 y with the mean age of 53 y. The BOC has a distinct tendency for occurrence in the mandible, anterior to the first molar. Clinically it may be asymptomatic although numerous are symptomatic and radiographically it is multilocular, often extending between the periapical regions of the related teeth as the present case. The differential diagnosis for multilocular radiolucencies includes Odontogenic keratocyst, Multicystic ameloblastoma, Odontogenic myxoma, Ameloblastic fibroma, Central odontogenic fibroma, and Intraosseous mucoepidermoid carcinoma [1–3]. The incisional biopsy was characteristic of BOC, but the presence of follicles in excisional biopsy specimen put us at the crossroads of BOC, multicystic ameloblastoma, unicystic ameloblastoma with mural follicular proliferations and cystic epithelium undergoing ameloblastomatous transformation. Hence, the whole specimen was processed and deeper sections of the incisional and excisional blocks were examined. Except for the five follicles identified initially, none of the slides showed the presence of follicles and moreover multicystic spaces were lined by flattened cells. And characteristic peripheral tall columnar cells with reverse nuclear polarity or cuboidal cells or central stellate reticulum like cells were not identified. To attribute it for a cyst undergoing ameloblastomatous transformation Vickers and Gorlin criteria was absent and to admit it for unicystic ameloblastoma characteristic epithelium was absent apart from it being multicystic radiographically and histologically. The histogenesis of both LPC and BOC includes a number of possible sources of odontogenic epithelium, including cell rests of dental lamina, epithelial rests of malassez and reduced enamel epithelium [4]. Wysocki et al., proposed that it arises from clear cell rests of dental lamina suggesting that Unicystic forms arise through cystic change in a single dental lamina rest and polycystic lesions develop from changes in several adjacent cell rests especially from the post functional cells of dental lamina [5]. Some postulated it to result from proliferation of rests of malassez in periodontal ligament and some thought it to be due to compartmentalization in LPC lumen while others state that it arises as a result of fusion of multiple independent LPCs developing in proximity. However now it is sturdily suggested that BOC is simply the polycystic variant of LPC developing through cystic transformation of multiple islands of dental lamina rests [4–6]. Till date there is no clear cut perception in literature to differentiate BOC and ameloblastoma, though cases of BOC with follicles are reported. In such cases along with clinical, radiological, and histopathological discriminating parameters the use of IHC staining was questionable. Hence we used CD56 and calretinin to differentiate them. Absence of expression of CD56 antigen and calretinin in the cystic lining of BOC possibly rules out ameloblastoma. CD56 expression was seen in the cytoplasm of peripheral cells of the follicles faintly. Altini et al., reported expression of calretinin in 81.5% of unicystic ameloblastomas and 93.5% of multicystic ameloblastomas whereas no expression was seen in residual cysts, dentigerous cysts, and odontogenic keratocysts except for a study by Piattelli et al., were calretinin positivity was seen in the proliferating epithelium in Odontogenic keratocyst [7–9]. Careful surgical excision is the treatment of choice for BOC and conservative enucleation is contraindicated as the recurrence rate in BOC is around 34% [1], which is due to the difficulty in removing or failure to remove the entire multilocular lesion during surgery. The long term follow-up is mandatory because of high recurrence rate [2,10]. Pathological categorization of a lesion as BOC is important mainly with regard to the treatment aspect and the follow-up period, as LPC can be treated by surgical enucleation and has very low recurrence rate. The treatment for ameloblastoma ranges from simple enucleation, curettage, chemical and electrocautery, radiation, combination of radiation and surgery to en bloc resection [11]. But the usual line of treatment in ameloblastoma is marginal resection with a recurrence rate of upto 15% which is much less when compared to BOC although both the lesions require a definite long term follow-up. The present case was followed up for every week for two months and later on monthly visit for a period of three years were instituted.
Conclusion
BOC with follicles should be differentiated from ameloblastomatous transformation of BOC using CD 56 and calretinin as the treatment options and recurrences rate of both vary apparently.
[1]. Méndez P, Junquera L, Gallego L, Baladrón J, Botryoid odontogenic cyst: clinical and pathological analysis in relation to recurrenceMed Oral Patol Oral Cir Bucal 2007 12(8):594-98. [Google Scholar]
[2]. Mori K, Tamura N, Tamura N, Shimada J, Botryoid odontogenic cyst: A case report with immunohistochemical aspectsAsian Journal of Oral and Maxillofacial Surgery 2011 23:31-34. [Google Scholar]
[3]. Santos Andrade PP DE, Valéria Souza Freitas SV, Freitas RDA, Pinto LP, Batista DSL, Botryoid odontogenic cyst: A clinicopathologic study of 10 casesAnnals of Diagnostic Pathology 2011 15:221-24. [Google Scholar]
[4]. Rajendran R, Sivapathasundaram B, Shafer’s Textbook of Oral pathology 2009 sixth editionUSAElsevier company [Google Scholar]
[5]. Shear Mervyn, Speight Paul, Cysts of the Oral and Maxillofacial Regions 2007 Fourth edition Blackwell Munksgaard company [Google Scholar]
[6]. Arora P, Kundendu AB, Nishanth G, Jamdade A, Kumar GR, Botryoid Odontogenic Cyst Developing from lateral periodontal cyst : A rare case and review on pathogenesisContemporary Clinical Dentistry 2012 3:326-29. [Google Scholar]
[7]. Altini M, Shear M, The Lateral periodontal Cyst : An UpdateJ Oral Pathol Med 1992 21:245-50. [Google Scholar]
[8]. Nuray E, Deviren AD, Tasman F, Zeybek D, Neural Cell Adhesion Molecule and Neurothelin Expression in Human AmeloblastomaJ Oral Maxillofac Surg 2001 59:900-03. [Google Scholar]
[9]. DeVilliers P, Liu H, Suggs C, Simmons D, Daly B, Zhang S, Raubenheimer E, Calretinin Expression in the Differential Diagnosis of Human Ameloblastoma and Keratocystic Odontogenic TumorAm J Surg Pathol 2008 32:256-60. [Google Scholar]
[10]. Gurol M, Burkes EJ, Jacoway J, Botryoid Odontogenic Cyst: Analysis of 33 CasesJ Periodontol 1995 66:1069-73. [Google Scholar]
[11]. Brad WN, Douglas D, Carl MA, Jerry EB, Oral and maxillofacial pathology 2002 second editionPhiladelphiaWB saunders company [Google Scholar]