Evaluation of Different Staining Techniques in the Diagnosis of Trichomonas vaginalis Infection in Females of Reproductive Age Group
Razia Khatoon1, Noor Jahan2, Haris Manzoor Khan3, Tamkin Rabbani4, Siraj Ahmad5
1 Assistant Professor, Department of Microbiology, Era’s Lucknow Medical College and Hospital, Lucknow, India.
2 Assistant Professor, Department of Microbiology, Era’s Lucknow Medical College and Hospital, Lucknow, India.
3 Professor, Department of Microbiology, Jawaharlal Nehru Medical College and Hospital, Aligarh, India.
4 Associate Professor, Department of Obstetrics and Gynaecology, Jawaharlal Nehru Medical College and Hospital, Aligarh, India.
5 Associate Professor, Department of Community Medicine, Teerthanker Mahaveer Medical College and Research Centre, Teerthanker Mahaveer University, Moradabad, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Noor Jahan, Department of Microbiology, Era’s Lucknow Medical College and Hospital, Lucknow-226003, India. E-mail : drnoorj@rediffmail.com
Background: Trichomonas vaginalis is a parasitic protozoan which causes most common non viral sexually transmitted disease trichomoniasis. Direct microscopic examination of vaginal fluid remains the most widely used diagnostic test. Although, wet mount examination is the most cost-effective diagnostic test, but it has low sensitivity resulting in under diagnosis of the disease. Therefore, to overcome this problem, various staining techniques like giemsa and acridine orange can be used along with wet mount examination for diagnosis of T. vaginalis infection.
Objective: The present study was done to evaluate the efficacy of Giemsa and Acridine Orange staining in comparison with wet mount examination for the diagnosis of vaginal trichomoniasis.
Materials and Methods: A total of 615 female patients of reproductive age group having vaginal discharge were included in the study and swabs containing vaginal fluids were taken to perform wet mount examination, giemsa staining and acridine orange staining.
Result: Trichomonas vaginalis infection was detected in 37 patients with maximum cases (6.0%) detected by acridine orange staining, followed by giemsa staining (4.9%), whereas, wet mount examination was able to detect only 4.1% cases. Wet mount examination gave a sensitivity of 67.6%, whereas, the sensitivity of giemsa staining and acridine orange staining was found to be 80% and 100% respectively.
Conclusion: Since the performance of both the staining techniques was found to be much better in comparison to wet mount examination, and they also detected several wet mount negative cases, they should be used as an adjunct to wet mount examination. This will also be beneficial to the overall health of the patient by early diagnosis and treatment of cases, thereby, reducing the development of associated morbidity.
Acridine orange staining, Giemsa staining, Trichomonas vaginalis, Wet mount
Introduction
Trichomonas vaginalis causes most common non-viral sexually transmitted disease, which has the world-wide annual incidence of more than 180 million cases per year [1]. Although the most common clinical presentation in females is vaginitis and foul smelling vaginal discharge, it has a varied range of presentation from asymptomatic infection to symptomatic diseases and complications in the form of endometritis, infertility, infection of adnexa and bartholin glands and enhanced risk of neoplastic transformation of cervical tissues [2]. In addition, there is also increased risk of acquiring other sexually transmitted diseases including human immunodeficiency virus infection [3–5].
The diagnosis of trichomoniasis has traditionally depended on the microscopic observation of motile protozoa from vaginal or cervical samples in females and urethral or prostatic secretions in males [6]. Currently, wet mount microscopy is the most rapid and widely used method for diagnosing trichomoniasis in resource-constrained settings [7]. However, it is a subjective test that requires experience and even in the hands of trained observers it is only 35 to 80% sensitive compared to culture [8]. This lack of sensitivity contributes to the under diagnosis of the disease. Also, since viable organisms are required, a delay in the transport of sample reduces motility of the organism and, consequently, diagnostic sensitivity [9]. This can be overcome by using staining techniques in adjunct to wet mount microscopy. Stained smears have the advantage that there can be considerable delay between preparation and staining and examination of the smear without loss of reliability in diagnosis, provided the smear has been adequately fixed. Various stains commonly used are Papanicolaou, Giemsa and other Romanowsky stains [10–12]. Besides these, a number of staining techniques using Acridine Orange, Leishman, Periodic Acid Schiff and Fontana staining methods have been used to improve the sensitivity of direct microscopy [13–16]. Giemsa stain, no doubt, is the most readily available stain in the average laboratory and the results, even in poorly prepared smear, justify its application. Certain features, such as; spindle shaped nucleus, the contours of the organism, and the cytoplasmic inclusions still make it possible to recognize and diagnose the organism easily. Acridine orange staining is a non-specific nucleic acid staining procedure which can be applied for fluorescence-based detection of T.vaginalis [17].
Hence, the present study was done to evaluate the efficacy of staining procedures like giemsa staining and acridine orange staining in comparison with wet mount examination for the diagnosis of Trichomonas vaginalis infection in females of reproductive age group.
Materials and Methods
A hospital based prospective study was done in the Department of Microbiology and outpatient department of Obstetrics and Gynaecology, Jawaharlal Nehru Medical College and Hospital, Aligarh, India, over a period of one year from May 2009 to April 2010. The study group comprised of 615 female patients of reproductive age group (15-45 years) with complaints of foul smelling vaginal discharge, pruritis, dyspareunia, dysuria, and pain in lower abdomen. The study was approved by the Institutional Ethics Committee. An oral informed consent was taken from each patient and two vaginal swabs were taken from posterior fornix. One was used to prepare wet mount and second was used to prepare smear for giemsa staining and acridine orange staining.
Wet Mount Examination
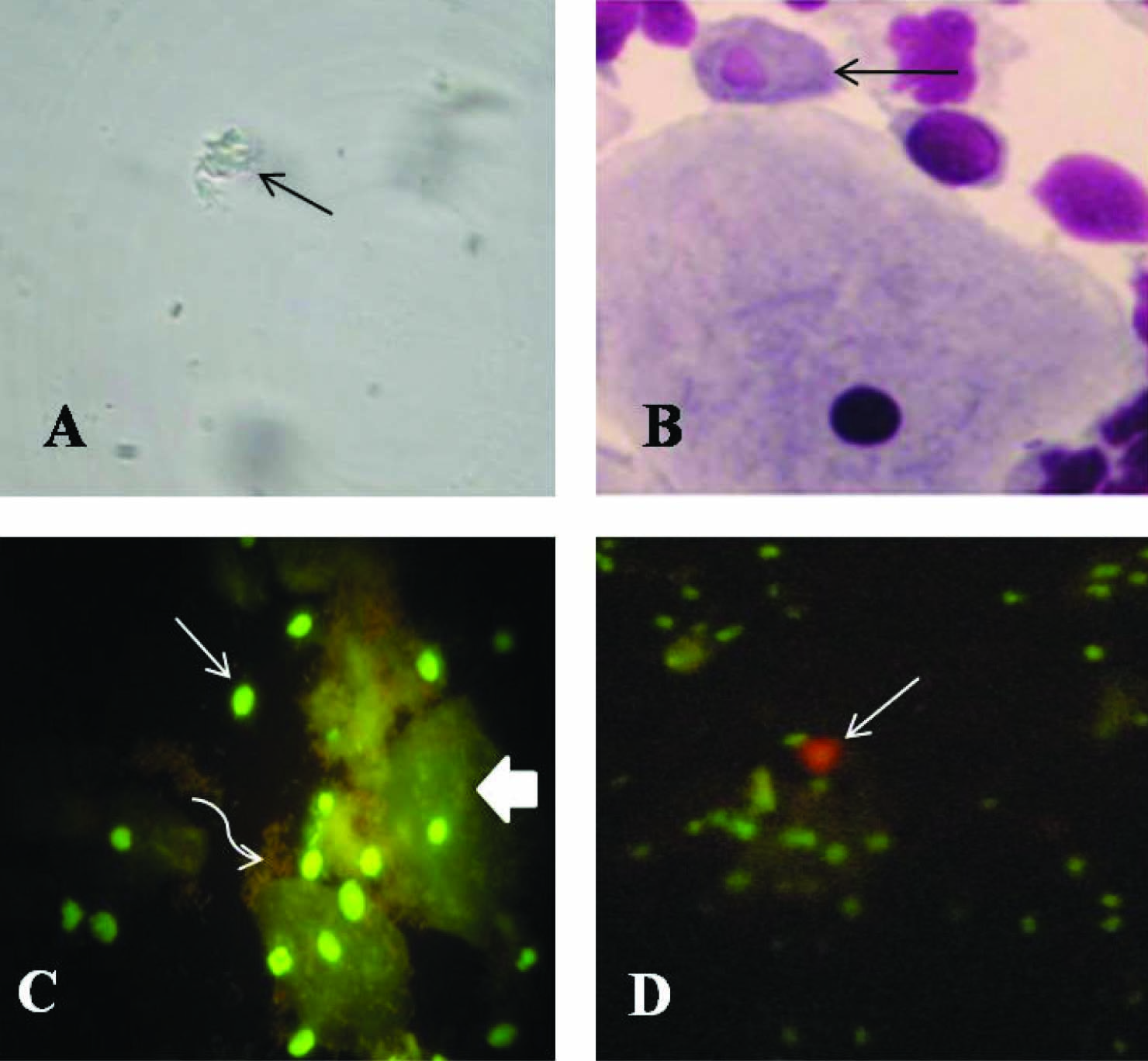
Wet mount was prepared using 0.85% physiological saline and examined with a light microscope at 10X and 40X [18]. The trichomonads were identified by their size (10-20 μm), round or oval shape, and characteristic quivering or twitching motility [Table/Fig-1a].
a)Wet mount showing Trichomonas vaginalis (thin arrow); b) Giemsa staining showing Trichomonas vaginalis (thin arrow); c) Acridine Orange staining showing epithelial cell (thick arrow), pus cell (thin arrow) and bacteria (curved arrow); d) Acridine Orange staining showing brick red coloured Trichomonas vaginalis (thin arrow)
Giemsa Staining
The prepared smear was fixed by immersion in methanol for one minute and allowed to dry. It was then stained with giemsa stain (Hi Media Laboratories, India), diluted 1 part to 19 parts of 1/15M phosphate buffer, pH 7.2 for 10 min and scanned for Trichomonas vaginalis at 100X magnification [19]. Both the internal and external structures of the organism were clearly visualized. The former stained dark blue with a red nucleus and the latter was sharply outlined, showing clearly the flagella and the undulating membrane [Table/Fig-1b].
Acridine Orange Staining
The prepared smear was fixed with methanol for 2-3 min and covered with freshly prepared acridine orange dye (Hi Media Laboratories, India) in a concentration of 5mg/ml which was then left at room temperature for 2 min. After being rinsed with distilled water, the slide was examined under a fluorescence microscope (with a 470-490 nm filter) at a magnification of 40X [20]. Epithelial cells fluoresced light green with a bright green nucleus. The nuclei of leukocytes (pus cells) fluoresced bright green and bacteria stained bright red [Table/Fig-1c]. The trophozoites of Trichomonas vaginalis were seen as characteristic brick red colour with a yellowish green nucleus [Table/Fig-1d].
Statistical Analysis
The collected data were analyzed by using SPSS Data Editor Software, version 16 (SPSS Inc, United States). Pearson’s Chi-square test was performed and p values <0.05 were considered statistically significant.
Results
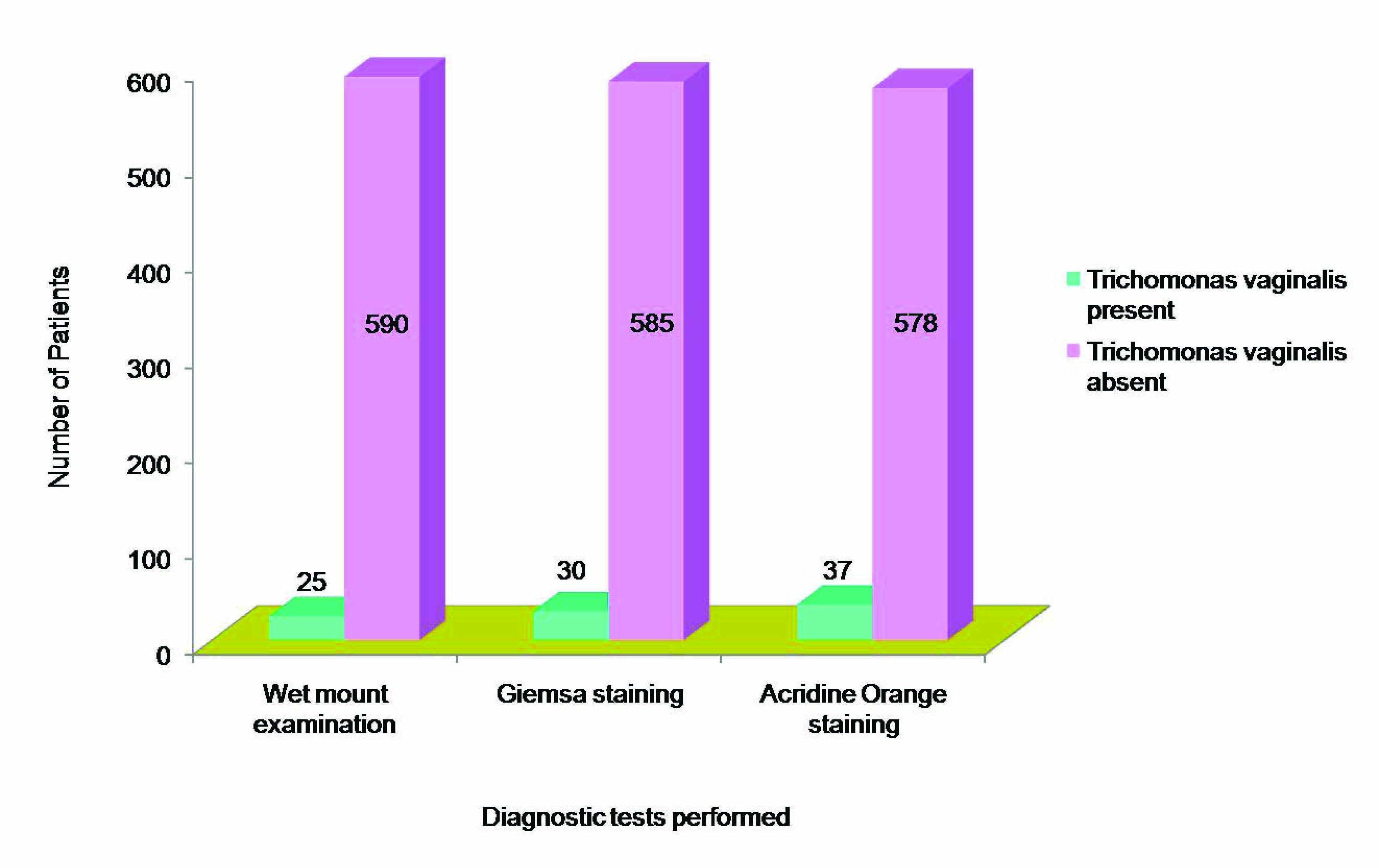
A total of 615 female patients of reproductive age group (15-45 years) were included in the study. The mean age of the patients was 30.56±0.34 years. Direct microscopic examination of smears from vaginal swab detected 37 positive cases of trichomoniasis. Wet mount examination for T. vaginalis was positive in 4.1% of cases, whereas, giemsa staining and acridine orange staining were positive in 4.9% and 6.0% of cases respectively [Table/Fig-2]. Out of total 37 positive cases, 25 cases were positive in wet mount examination. The sensitivity of wet mount examination was found to be 67.6%.
Detection of Trichomonas vaginalis by wet mount examination and staining techniques
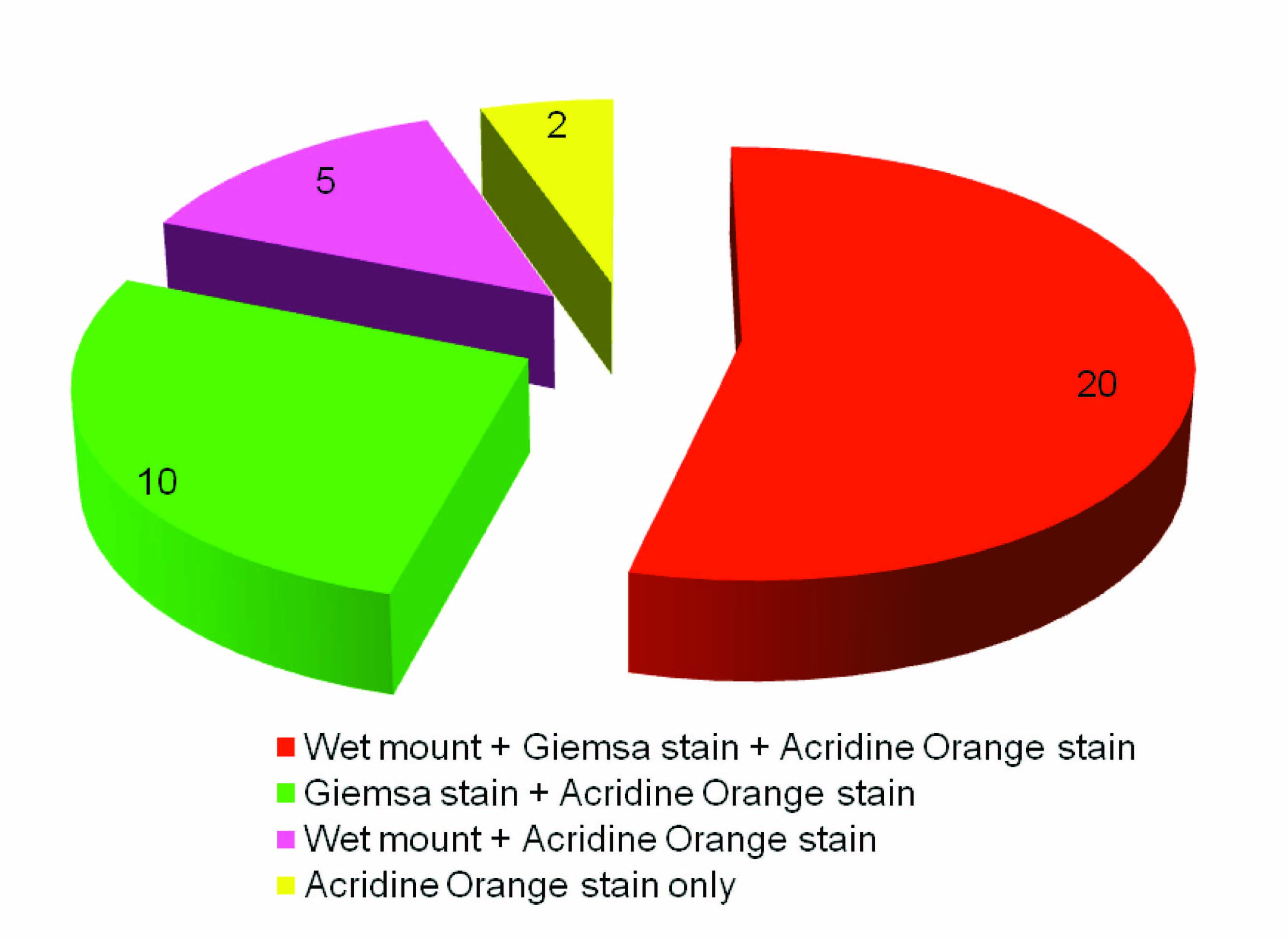
A correlation was done between the different diagnostic tests performed and it was found that out of 37 positive cases, giemsa staining detected 10 wet mount negative cases, whereas, acridine orange staining detected 12 wet mount negative cases along with 7 giemsa negative cases [Table/Fig-3].
Correlation between different diagnostic tests performed for diagnosing Trichomonas vaginalis infection
The efficacy of staining techniques was determined in comparison with wet mount examination and sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of giemsa staining and acridine orange staining were calculated [Table/Fig-4]. Pearson’s Chi-square test was done and the difference in the sensitivity of giemsa staining and acridine orange staining in comparison with wet mount examination was found to be statistically significant (p<0.001).
Efficacy of giemsa staining and acridine orange staining in comparison with wet mount examination
| Staining techniques performed | Sensitivity (%) | Specificity (%) | Positive predictive value (%) | Negative predictive value (%) |
|---|
| Giemsa staining | 80.0 | 98.3 | 66.7 | 99.1 |
| Acridine Orange staining | 100 | 98.0 | 67.6 | 100 |
Discussion
Wet mount examination is the most frequently used method for diagnosis of trichomoniasis in women. In our study, out of the 615 patients tested, 25 (4.1%) cases were positive for T vaginalis infection by wet mount examination with sensitivity of 67.6%. It has been reported that the sensitivity of wet mount examination ranges between 35-80% depending on the technical expertise of the observer [8]. In one of the study it was shown that the sensitivity and specificity of direct wet mount microscopy was 95.83% and 100% respectively, in comparison to culture [21]. Although, positive wet mount is diagnostic, a negative test cannot exclude trichomoniasis because of low sensitivity [22]. It is reported that a minimal concentration of 104 organisms per millilitre of vaginal fluid is necessary for the identification of this protozoan by wet mount as low number of trichomonas can be easily missed when they are in the presence of large number of leukocytes [23]. Since the protozoa lose their distinctive motility on cooling to room temperature, a microscope and an experienced microscopist must be readily available in the clinical setting and the specimen should be examined as quickly as possible [9].
The performance of giemsa staining was good and it was able to detect Trichomonas vaginalis in 30 (4.9%) out of 615 patients, with a sensitivity of 80% and specificity of 98.3%. Studies done by different workers have reported varying range of sensitivity (41-56%) of giemsa stained smears for the diagnosis of Trichomonas vaginalis infection [19,24,25]. In one study it was reported that giemsa staining had high sensitivity (100%) and specificity (99.69%) compared to culture. The high sensitivity (100%) reported in their study was attributed to centrifugation of sample prior to preparation of smears which led to concentration of large number of parasites [21]. In set up lacking immediate microscopic facilities giemsa staining is very useful, where prepared and fixed smears can be transported to the laboratory for diagnosis. Also, with large number of patients attending gynaecological outpatient department, an immediate examination of a vaginal swab is virtually impossible. Unlike, wet mount examination, delay in transport has no significant impact on its reliability for diagnosing Trichomonas vaginalis. However, it is time consuming and needs technical expertise.
Acridine orange staining was positive in 37 (6.0%) out of 615 patients included in the study, with sensitivity and specificity of 100% and 98.0% respectively. Whereas, in one study acridine orange staining showed a high sensitivity and specificity of 71.43% and 99.44% respectively, in another study its sensitivity was only 47.5% in comparison to culture [25,26]. Also, it has been reported that the sensitivity of acridine orange staining is high (67%) in women with T.vaginalis infection alone, and low (53%) in women with multiple infections [17].
In the present study, maximum number of cases of vaginal trichomoniasis was detected by acridine orange staining. This is in agreement with a previously done study which showed that the infection rate detected by acridine orange staining was higher than either culture or other vaginal swab examinations [19]. Although acridine orange staining is easy to perform, rapid and screening of the vaginal smears is done in less time, the major disadvantage of this technique is that it requires special microscopic facilities, trained personnel and availability of the dye. Also, the smears lose their fluorescence with the passage of time, so permanent record is not possible. However, the rapidity, ease and reliability of acridine orange staining justify its use in routine laboratory diagnosis of trichomonal infection.
Conclusion
Although wet mount examination is the most commonly used test in routine diagnosis of Trichomonas vaginalis infection, but staining techniques should be used as an additional diagnostic test in order to diagnose cases missed either due to unavailability of immediate microscopic facility or delay in the transport of samples to the laboratory for culture. Acridine orange staining was found to be the best microscopic method when compared with wet mount examination and giemsa staining. Hence, it should be used in routine diagnosis of Trichomonas vaginalis infection in places where fluorescent microscopic facility is available. This will also provide rapid screening of vaginal smears in patients suffering from vaginal discharge and thus, help in early diagnosis and prompt treatment of patients. As giemsa staining performed well in comparison to wet mount examination and can easily be done in routine, it should be used as an adjunct to wet mount microscopy in small setups lacking facilities for fluorescent microscopy. This will ultimately prove beneficial to patients in reducing morbidity and associated adverse health outcomes in the form of pelvic inflammatory diseases and increased risk of acquiring other sexually transmitted diseases including human immunodeficiency virus infection.
[1]. Jamali R, Zareikar R, Kazemi A, Yousefee S, Ghazanchaei A, Estakhri R, Diagnosis of Trichomonas vaginalis infection using PCR method compared to culture and wet mount microscopyInt Med J Malaysia 2006 5:1-8. [Google Scholar]
[2]. Chinyere OE, Sabinus AE, Chinedu IJ, Okoro CN, Chidiebube NA, Chinwe OJ, Prevalence of Trichomonas vaginalis among pregnant women in Abakaliki, Ebonyi stateInt J Curr Res 2010 11:11-15. [Google Scholar]
[3]. Jongh MD, Roux ML, Adam A, Caliendo AM, Hoosen AA, Co-infection with Neisseria gonnorrhoeae, Chlamydia trachomatis and Trichomonas vaginalis in symptomatic South African Men with Urethritis: Implications for Syndromic ManagementThe Open Trop Med J 2009 2:13-16. [Google Scholar]
[4]. Shafir SC, Sorvillo FJ, Smith L, Current issues and considerations regarding trichomoniasis and human immunodeficiency virus in African-AmericansClin Micro Rev 2009 22(1):37-45. [Google Scholar]
[5]. Rathod SD, Krupp K, Klausner JD, Arun A, Reingold AL, Madhivanan P, Bacterial vaginosis and risk for Trichomonas vaginalis infection: A longitudinal analysisSex Transm Dis 2011 38(9):882-86. [Google Scholar]
[6]. Alcamo IE, Fundamentals of microbiology 2000 BostonJones and Bartlett Publishers:486-87. [Google Scholar]
[7]. Madhivanan P, Li T, Trammell S, Desai C, Srinivas V, Arun A, Performance of the OSOM Trichomonas Rapid Test for diagnosis of Trichomonas vaginalis infection among women in Mysore, IndiaSexual Health 2013 10:320-24. [Google Scholar]
[8]. Beverly AL, Vengtarik LM, Cotton B, Schwebke JR, Viability of Trichomonas vaginalis in transport mediumJ Clin Microbiol 1999 37:3749-50. [Google Scholar]
[9]. Clark DH, Solomons E, An evaluation of routine culture examination for Trichomonas vaginalis and CandidaAm J Obstet Gynecol 1959 78:1314-9. [Google Scholar]
[10]. Hughes HE, Gordon AM, Barr GTD, A clinical laboratory study of trichomonias of the female genital tractJ Obstet Brit Cwlth 1966 73:821-27. [Google Scholar]
[11]. Freeman F, A modified staining technique for Trichomonas vaginalisS Afr Med J 1958 32:1235 [Google Scholar]
[12]. Lowe GH, The laboratory diagnosis of trichomoniasisIn Laboratory Diagnosis of Venereal Disease 1972 LondonPublic Health Laboratory Service Monograph Series No. 1:40-42. [Google Scholar]
[13]. Fripp PJ, Mason PR, Super H, A method for the diagnosis of Trichomonas vaginalis using acridine orangeJ Parasitol 1975 61:966-67. [Google Scholar]
[14]. Levett PN, A comparison of five methods for the detection of Trichomonas vaginalis in clinical specimensMed Lab Sci 1980 37:85-88. [Google Scholar]
[15]. Rodriguez-Martinez HA, De la Luz Rosales M, Gallaso de Bello L, Ruiz-Moreno JA, Adequate staining of Trichomonas vaginalis by McManus’ periodic acid-Schiff stainAm J Clin Pathol 1973 59:741-46. [Google Scholar]
[16]. Nagesha CN, Ananthkrishna NC, Sulochana P, Clinical and laboratory studies on vaginal trichomoniasisAm J Obstet Gynecol 1970 106:933-35. [Google Scholar]
[17]. Bickley LS, Krisher KK, Punsalang A Jr, Trupei MA, Reichman RC, Menegus MA, Comparison of direct fluorescent antibody, acridine orange, wet mount, and culture for detection of Trichomonas vaginalis in women attending a public sexually transmitted diseases clinicSex Transm Dis 1989 16(3):127-31. [Google Scholar]
[18]. Cheesbrough M, District Laboratory Practice in Tropical Countries, Part 2 2000 Cambridge University Press [Google Scholar]
[19]. Mason PR, Super H, Fripp PJ, Comparison of four techniques for the routine diagnosis of Trichomonas vaginalis infectionJ Clin Path 1976 29:154-57. [Google Scholar]
[20]. Schee CVD, Belkum AV, Zwijgers L, Brugge EVD, O’Neill EL, Luijendijk AD, Improved Diagnosis of Trichomonas vaginalis Infection by PCR Using Vaginal Swabs and Urine Specimens Compared to Diagnosis by Wet Mount Microscopy, Culture, and Fluorescent StainingJ Clin Microbiol 1999 37(12):4127-30. [Google Scholar]
[21]. Fernando SD, Herath S, Rodrigo C, Rajapakse S, Improving diagnosis of Trichomonas vaginalis infection in resource limited health care settings in Sri LankaJ Glob Infect Dis 2011 3(4):324-28. [Google Scholar]
[22]. Swygard H, Sena AC, Hobbs MM, Cohen MS, Trichomoniasis: clinical manifestations, diagnosis and managementSex Transm Infect 2004 80:91-95. [Google Scholar]
[23]. Krieger JN, Tam MR, Stevens CE, Nielsen IO, Hale J, Kiviat NB, Diagnosis of trichomoniasis: Comparison of conventional wet-mount examination with cytologic studies, cultures, and monoclonal antibody staining of direct specimensJAMA 1988 259(8):1223-27. [Google Scholar]
[24]. Agarwal S, Sharma V, Sarin R, Reproductive tract infections in women- Prevalence, HIVseropositivity and role of conventional methods in diagnosisIndian J Sex Transm Dis 2005 26(2):73-77. [Google Scholar]
[25]. Radonjic IV, Dzamic AM, Mitrovic SM, Arsenijevic VSA, Popadic DM, Zec IFK, Diagnosis of Trichomonas vaginalis infection: The sensitivities and specificities of microscopy, culture and PCR assayEur J Obstet Gynecol Repro Biol 2006 126(1):116-20. [Google Scholar]
[26]. Cevahir N, Kaleli I, Kaleli B, Evaluation of direct microscopic examination, acridine orange staining and culture methods for studies of Trichomonas vaginalis in vaginal discharge specimensMikrobyol Bul 2002 36:329-35. [Google Scholar]