Contrast-induced nephropathy (CIN) is an important complication of both diagnostic and therapeutic cardiac catheterization procedures and is associated with prolonged hospitalization, morbidity, mortality with worse short-term and 1-year survival [1]. It is the third most common cause of hospital-acquired acute renal failure and hypotension after a surgery or a procedure [2].The most commonly used definition of CIN after cardiac catheterization is a rise in serum creatinine levels of 0.5mg/dl, or a 25% increase from baseline [3].The true incidence of CIN is difficult to assess because of difference in the clinical outcome of high risk patients, types of contrast media used and also because of preventive measures. However, the scope of the problem is very large. The commonly used dyes are low- osmolar, non-ionic, monomer Iohexol (Omnipaque) and iso- osmolar, non-ionic, dimer, Iodixanol (Visipaque).
In view of this drastic & impending future impact on clinical outcomes, CIN has been the subject of extensive investigation, leading to the recognition of various risk factors. Various hypotheses have been proposed to explain the underlying disease process which has led to the development of multiple strategies to treat and prevent CIN [4]. Increasing data from various observational studies points towards uric acid being a risk factor for various renal disorders. In the last decades there has been a reappraisal of the relationship between elevated urate serum levels and an increased risk of renal disease [5–9]. The designation of CIN and its preventive strategies in major studies has varied markedly, making it exceedingly difficult to perform cross-study comparisons and form conclusions. The risk of CIN is < 10% but factors such as the stability of the patient's condition, the presence of shock, acute renal insufficiency and cardiomyopathy significantly increase the risk. Of these, preexisting renal failure particularly in a patient with diabetes and a history of prior anaphylactic reaction to contrast medium require special attention before coronary angiography to reduce the risk of subsequent complications.
A number of CIN therapies have been investigated. Short term studies and increasing data points towards uric acid being a risk factor as well as a biomarker for renal and various metabolic syndromes [10,11]. Further it has indicated that xanthine oxidase inhibitor like allopurinol has proved to be effective in preventing the above mentioned problems. Allopurinol has recently emerged as a rescuer in various cardio-renal problems for its pleiotropic effects.
Materials and Methods
This is a prospective, open label, randomized, controlled, interventional study conducted in the Department of Cardiology, GSL Medical College & Hospital, Rajahmundry, Andhra Pradesh, India, with due permission of the superintendent in charge of the hospital and institutional ethics committee. Five-hundred patients who underwent coronary angiography over the period from March 2012 to December 2012 were selected keeping in mind the inclusion and exclusion criteria. All the patients provided written informed consent for the procedures and the test drug. The detailed history, complete clinical, laboratory (biochemical parameters including electrolytes, renal & liver function tests), ECG and interventional data of all the above mentioned patients were obtained from the departmental data base and recorded in a Performa. Pre-procedural estimated risk of acute renal injury (referred to as mehran score) was calculated according to Mehran et al., [12].
Inclusion Criteria
All patients willing to undergo angiography and angioplasty with or without risk factors and patients who received maximum or less than maximum permissible dose of the dye calculated from 5x bodyweight (kg)/ serum creatinine in mg% were enrolled for the study [13].
Exclusion Criteria
Patients who received more than the maximum permissible dose of the dye, patients who were and continuing on any nephrotoxic drugs, patients already suffering from gout or serum uric acid levels >10mg/dl, any previous hypersensitivity or intolerance to allopurinol, congestive heart failure or ejection fraction < 40% and inability to give consent were excluded from the study.
Study Design
The 500 patients who underwent coronary angiography with either iohexol (omnipaque, each ml containing iohexol usp 1680 equivalent to iodine 350 mg) or iodixanol (visipaque, ml containing iodixanol usp 625 mg equivalent to iodine 320 mg) from GE were allocated to the following protocol for further study. Category 1 patients (275) were angiography positive (angiography proven significant cardiovascular disease needing revascularisation) and category 2 patients (225) were angiography negative (angiography showing normal or insignificant stenosis needing medical management). Further category 2 patients (225, angiography, negative) were discharged without further interventions.
Overall 56 patients developed contrast induced nephropathy (CIN) and 444 patients did not develop CIN. All the category 1 patients (275) underwent coronary angioplasty.
The patients (56) who developed CIN underwent coronary angioplasty after their serum creatinine (Scr) returned to normalcy. The angiography positive patients underwent a repeat laboratory and clinical evaluations and were randomised to the contrast dye omnipaque and visipaque with the same specifications as mentioned above during the angioplasty procedure. .To prevent further CIN the patients were pre and post treated with hydration and drugs [Table/Fig-1].
Flow diagram of the phases of the study, no.of patients assessed for eligibility and actually enrolled are shown
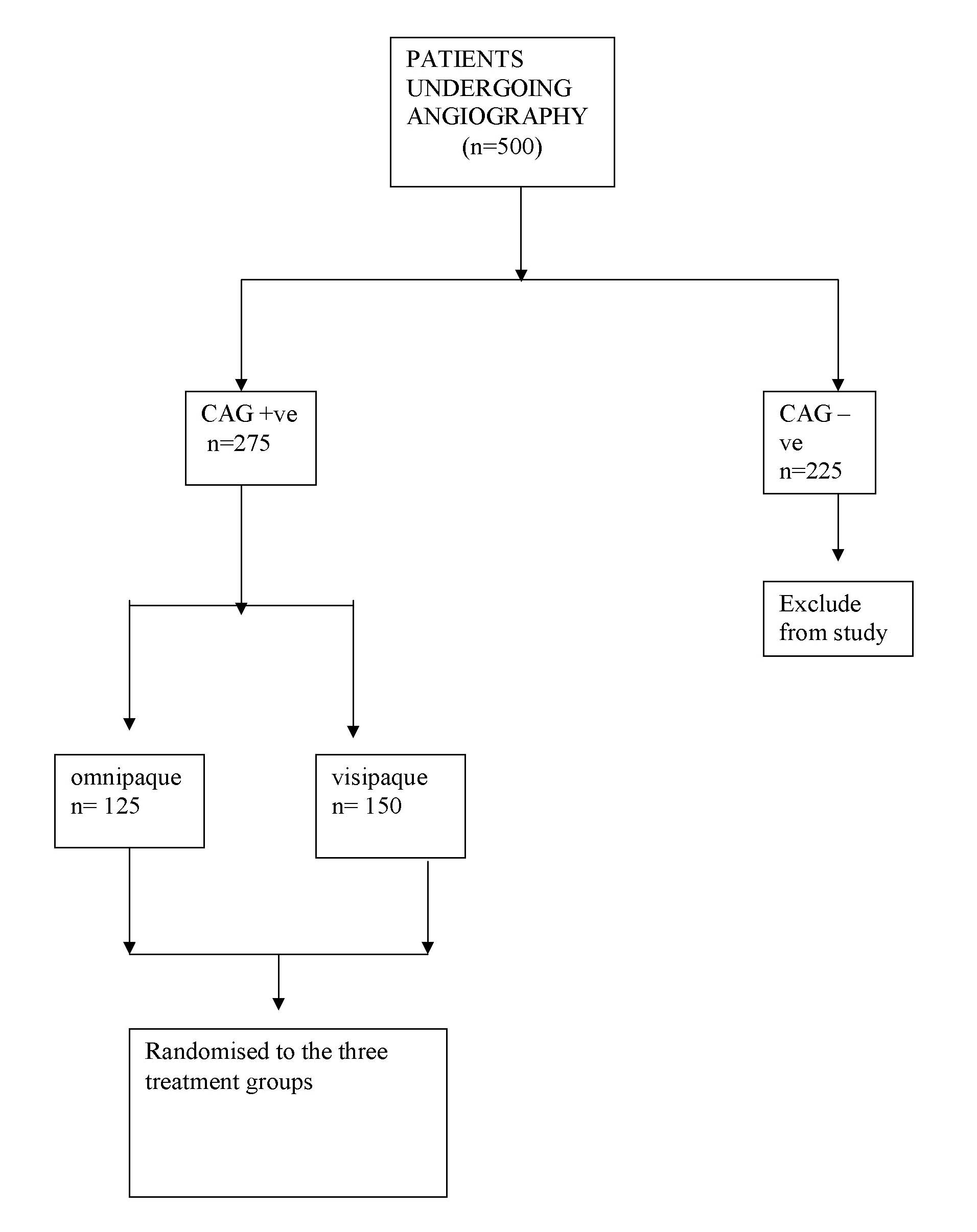
Patients who received omnipaque (125) were grouped as follows:
Group 1, Control (n=40) = Patients who were pre & post treated (12 hrs) with 0.9% saline 1ml/kgbw/min (max of 100 ml/hr) i.v. infusion.
Group 2 (n=40) = Patient who were pre and post treated (12 hrs) with 0.9% saline as above plus n-Acetyl cysteine (600 mg bd).
Group 3 (n=45) = Patient who were pre and post treated (12 hrs) with 0.9% saline as above plus Allopurinol (300mg/day).
Patients who received visipaque (150) were grouped similarly as those who received omnipaque but number of patients in each group were 50 (n=50).
Any incidence of urinary retention, back pain, bradycardia, hypotension, transient drop in oxygen saturation was noted for all the 500 patients.
The serum creatinine (Scr) and blood urea levels were recorded at interval of 24 hours for all the patients by evaluating these levels daily at a fixed time till the day 5 of the coronary angioplasty. The changes in the levels of the above renal parameters were recorded among the three treatment groups in patients receiving either of the two dyes and comparison made and represented in a tabular and diagrammatic form.
Study and Points
The primary end point was incidence of CIN in the two contrast media groups, change in the Serum creatinine levels, Blood urea levels, estimated glomerular filtration rate (e-GFR) within 48 h of cardiac catherisation in the three treatment groups.
Statistical Analysis
Continuous variables were reported as median (interquartile range) and were compared using non-parametric (Mann-Whitney U-test). Categorical variables were expressed as percentages and counts and compared using Chi-square test. Unpaired t-test was applied when comparing baseline pre procedure levels of treatment groups with post procedure levels at 1st day. Statistical comparison amongst the control and treatment groups was done by One Way Analysis of Variance (ANOVA) and for multiple comparisons vs. control group by Dunnett’s test.
Results
Primary Endpoints
Incidence of CIN and Other Side Effects: Our study showed an overall incidence of CIN in 10.6 % of the patients from a total of 500 patients who underwent angiography. A total of 275 patients were included in the final study for evaluating the effects of preventive treatments and CIN developed in 17.8% (49/275). The demographic and clinical characteristics of the patients in both the groups (Omnipaque and Visipaque) are comparable as shown in [Table/Fig-2].
Baseline characteristics of patients enrolled In the study
| Characteristics | Overall (n=500) | Omnipaque (n=300) | Visipaque (n=200) | p value |
|---|
| Age, years | 65 (62-71) | 66(61-70) | 68(63-71) | 0.3 |
| Female | 110 (22) | 75 (21 ) | 35(23) | 0.3 |
| BMI, kg/m2 | 28(25-31) | 27(26-30) | 28(26-31) | 0.35 |
| Smoking | 210(42) | 145(41) | 65(45) | 0.5 |
| Hypertension | 286(57) | 196(56) | 90(60) | 0.7 |
| Diabetes | 197(39 ) | 128(37) | 69 (46) | 0.2 |
| Dyslipidemia | 277(55) | 188(53.7) | 89(59) | 0.8 |
| Previous coronary episodes | 101(20 ) | 71(20) | 30 (20) | 0.6 |
| LVEF | 0.57(0.49-0.60) | 0.56(0.51-0.59) | 0.57(0.50-0.58) | 0.5 |
| Mehran score | 10(4-12) | 10(3-13) | 11(6-12) | 0.4 |
| Baseline serum creatinine, mg/dl | 0.9(0.9-1.2) | 1.0(0.9-1.3) | 1.1(0.9-1.2) | 0.1 |
| Baseline BUN, mg/dl | 23(18.8-24.9) | 22(18.7-27.2) | 24(19.2-25.5) | 0.7 |
| Baseline e-GFR, ml/min/1.73 m2 | 77(65-90) | 83(62-89) | 78(70-93) | 0.5 |
Values are median (interquartile range) or count (%): of BMI = Body mass index:
LVEF= Left ventricular ejection fraction:
BUN = Blood urea nitrogen
e-GFR = Estimated glomerular filtration rate
Incidence of urinary retention (70/300), backpain (95/300), bradycardia (14/300), hypotension (16/300), transient drop in oxygen saturation (21/300) was more common in patients receiving omnipaque and allergic reactions in the form of skin rashes were equal in both the groups [Table/Fig-3]. So it is concluded that Visipaque was safer in our study population.
Incidence of adverse drug effects and reactions among patients Receiving either of the two contrast media, Values are presented as counts (%)
| Group | Urinary Retention Needing cathet-erization | Back pain | Brady-cardia | Hypot-ension | Transient drop in Oxygen saturation | Allergic reactions Pruritus/rashes |
|---|
| Omnipaque Group(n=300) | 70(23.3) | 95(31.7) | 14(4.7) | 16(5.3) | 21(7) | 20(6.7) |
| Visipaque Group(n=200) | 30(15) | 32(16) | 8(4) | 8(4) | 8(4) | 18(9) |
| p-value | < 0.05 | < 0.01 | ns | < 0.05 | < 0.01 | ns |
Effect of Saline Hydration
Out of the 40 patients receiving saline hydration 16 patients in the omnipaque group showed a rise in the serum creatinine (Scr) levels > 25% from the baseline (-1 to 1st day, 0.30±0.07). A further rise (0.15±0.01) was seen from the 1st to the 3rd day and finally a decrease was observed from the 3rd to 5th day (-0.22±0.10). A similar trend was observed for the blood urea levels (13.2±2.3, 1.4±0.05, -2.6±0.9) [Table/Fig-4].
Creatinine and blood urea levels in patients receiving low osmolar omnipaque (iohoxenol) & Visipaque (iodixanol)
| Omnipaque (Iohoxenol) | Visipaque (Iodixanol) |
|---|
| Change In Serum Creatinine (mg/dl) | Group 1 (control) 0.9% saline hydration 1ml/kg/hr (n=40) | Group 2 Saline hydration plus Acetylcysteine 600 mg BD (n=40) | Group 3 Saline hydration plus allopurinol 300mg/day (n=45) | Group 1 (control) 0.9% saline hydration 1ml/kg/hr (n=50) | Group 2 Saline hydration plus Acetylcysteine 600 mg BD (n=50) | Group 3 Saline hydration plus allopurinol 300 mg/day (n=50) |
|---|
| -1 to 1st day | 0.30± 0.07 ** | 0.23±0.07 * a | 0.18±0.02 * b | 0.38± 0.10** | 0.31±0.08 ** | 0. 22±0.02 * b |
| 1st day to 3rd day | 0.15±0.06 | - 0.10±0.06 b | - 0.16±0.04 c | 0.18±0.01 | 0.09± 0.01 b | - 0.08± 0.02 c |
| 3rd day to 5th day | - 0.22± 0.10 | - 0.12± 0.06 b | -0.19±0.02 c | - 0.10± 0.02 | - 0.08± 0.01 b | - 0.12±0.01 c |
| Change In Blood Urea Levels (mg/dl) |
| -1 to 1st day | 13.2± 2.3** | 10.5± 1.8 * a | 9.4± 0.9 * b | 17.2± 4.3** | 16.5± 3.8 ** ns | 17.4± 3.6 ** ns |
| 1st day to 3rd day | 1.4± 0.05 | 0.9± 0.06 a | - 1.3± 0.07 c | 1.4± 0.07 | 0.9± 0.07 a | - 1.3± 0.03 c |
| 3rd day to 5th day | - 2.6± 0.9 | - 2.8± 1.0 ns | - 4.2± 1.6 c | - 1.6± 0.05 | - 3.9± 0.9 b | - 3.8± 0.9 b |
Results are mean ± SD in each group. Values include reduction/ elevation of levels compared to levels at the respective day comparisons are made between basal level and treatment groups at day 1 unpaired t test) *, ** p< 0.05, 0.01 respectively. Comparisons are made between saline control and treatments groups at respective days (one-way ANOVA ) a, b, c p< 0.05, 0.01, 0.001 respectively. Overall significance of difference in levels between treatment groups measured by dunnett’s test. (p< 0.05)
In patients receiving the visipaque dye 15 out of 50 patients showed a rise in Scr levels > 25% from -1 to 3rd day (0.38±0.10, 0.18±0.01) and further decreased from the 3rd to the 5th day (-0.10 ±0.02). A similar trend was observed for blood urea levels (17.2±4.3, 1.4±0.07, -1.6±0.05) [Table/Fig-4].
Effect of Saline Hydration Plus N-Acetylcysteine
In patients receiving Omnipaque a rise in Scr >25% from baseline was observed in only 8 out of 40 patients, 16 patients showed <25% rise (0.23±0.07) from -1 to 1st day. Further a fall in Scr levels was observed from 1st day to 5th day (-0.10±0.06, -0.12±0.06 respectively). Blood urea showed a rise from 1st to 3rd day and a fall from the end of 3rd day [Table/Fig-4].
In patients receiving visipaque dye a rise in Scr was observed > 25% from baseline in 10 out of 50 patients from -1 to 3rd day (0.31±0.08, 0.09±0.01). A similar trend was observed for the blood urea levels (16.5±3.8, 0.9±0.07, -3.9±0.9) [Table/Fig-4].
Effect of Saline Hydration Plus Allopurinol
In patients receiving omnipaque dye 30 out of 45 patients showed a rise in Scr levels < 25% from baseline (-1 to 1st day, 0.18±0.02) . Further a fall in Scr was observed from the end of 1st to the 5th day (- 0.16±0.04, -0.19±0.02). A rise in blood urea levels was observed in 6 out of 8 patients from -1 to 1st day (17.4±3.6) but a fall from the end of 1st to 5th day was observed (-1.3±0.03, -3.8±0.9) shown in [Table/Fig-4]. In patients receiving visipaque a similar trend of rise and fall of Scr and blood urea was observed [Table/Fig-4].
Comparison of The Three Treatment Groups
Omnipaque Group
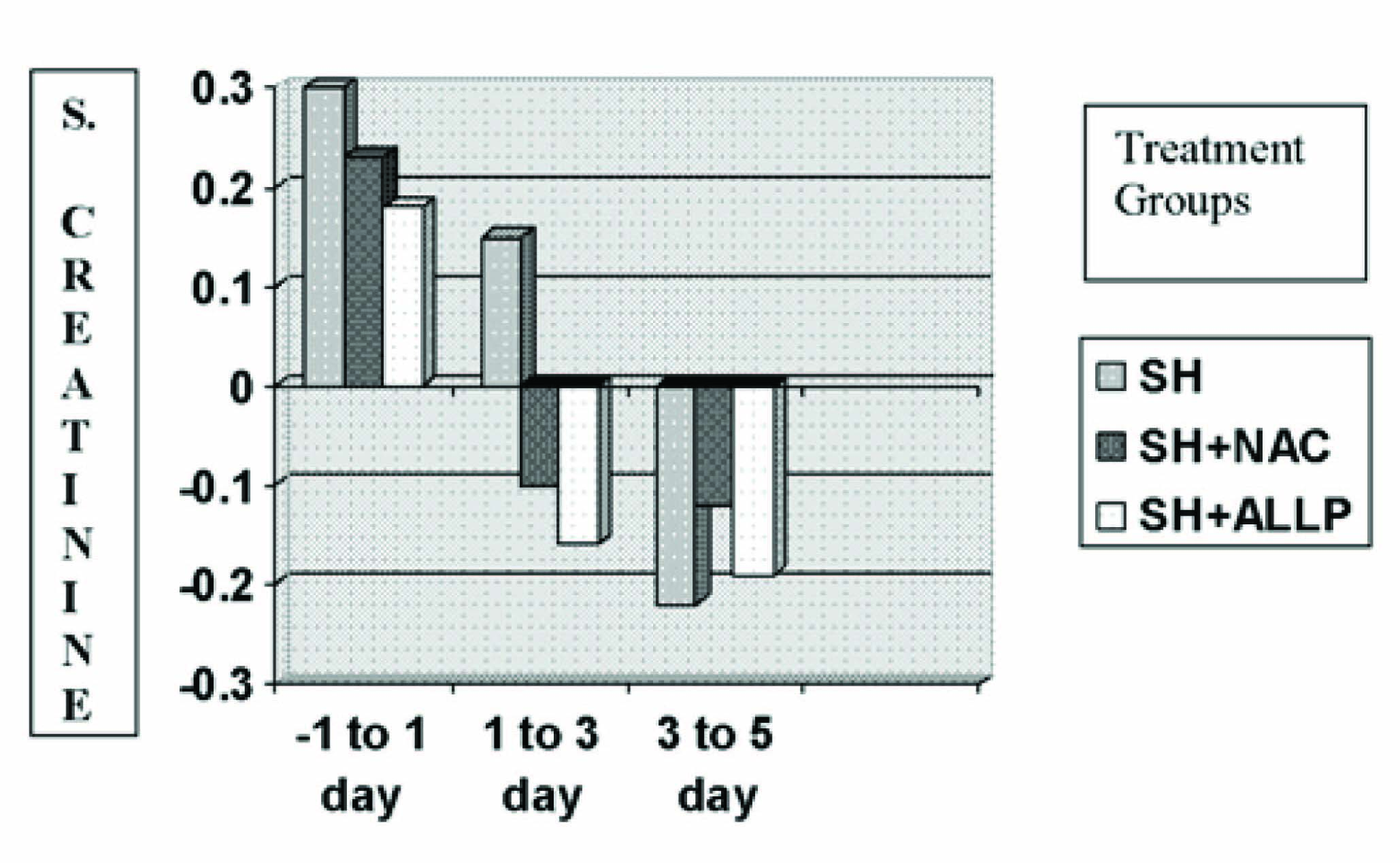
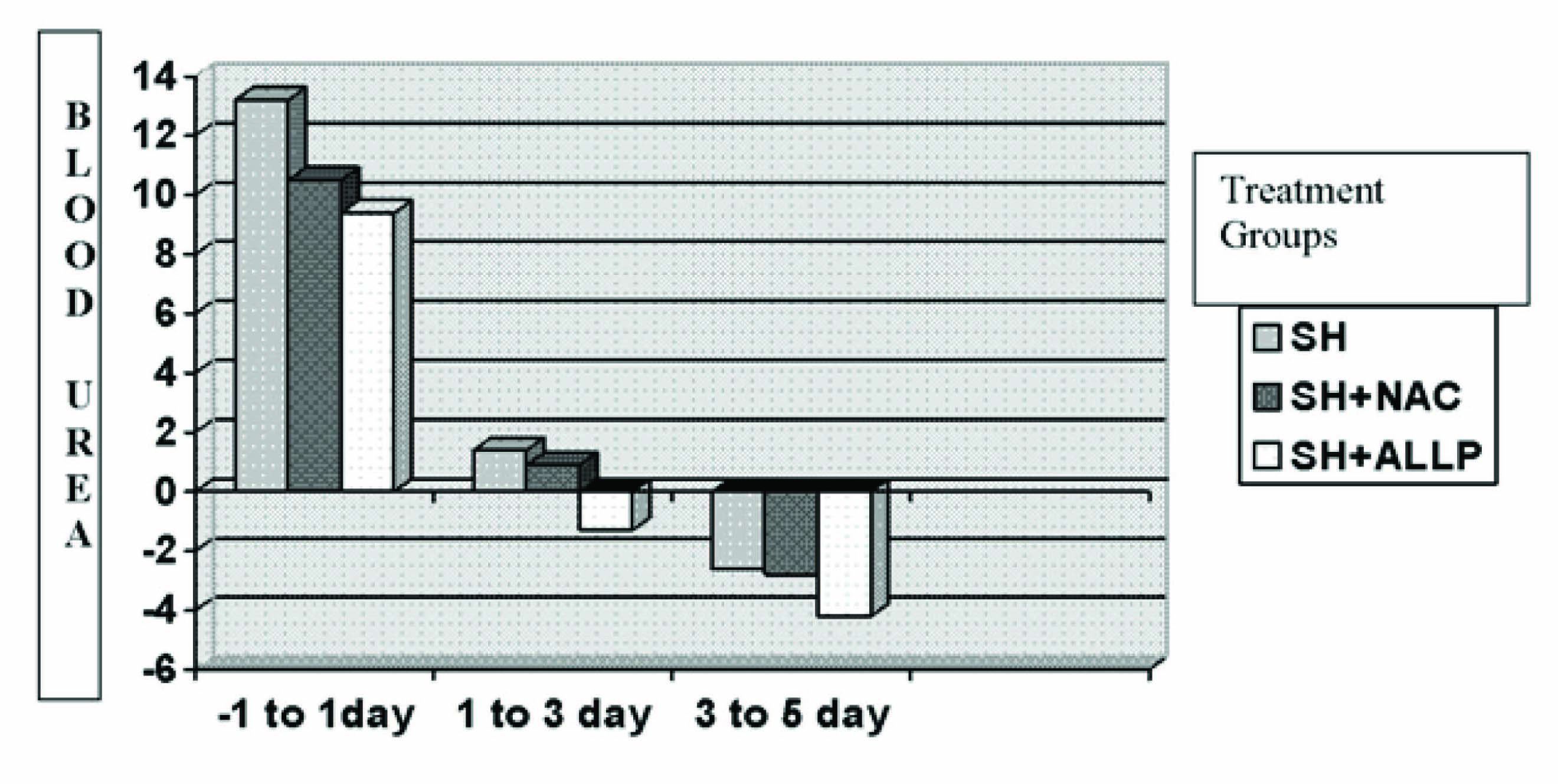
Rise in Scr in group 2 & 3 was significantly less than in group 1 compared to the baseline levels at -1 day (p <.05), similarly rise in Scr levels in group 2 & 3 when compared to control was also significantly less (p<0.05, 0.01). Fall in Scr levels was more significantly observed in group 3 (p<.0001) than in group 2 (p <0.01). Rise in blood urea levels when compared to basal levels was significantly less in group 2 & 3 (p<.05) than in group 1 (p <0.01). A fall in blood urea when compared to control group 1 was highly significant in group 3 from the 1st to 5th day (p < .001) than in group 2 (p=ns) [Table/Fig-5a and 5b].
Change in the levels of serum creatinine(mg/dl) in the three treatment groups in patients receiving omnipaque dye
Change in the levels of blood urea (mg/dl) in the three treatment groups in patients receiving omnipaque dye
Visipaque Group
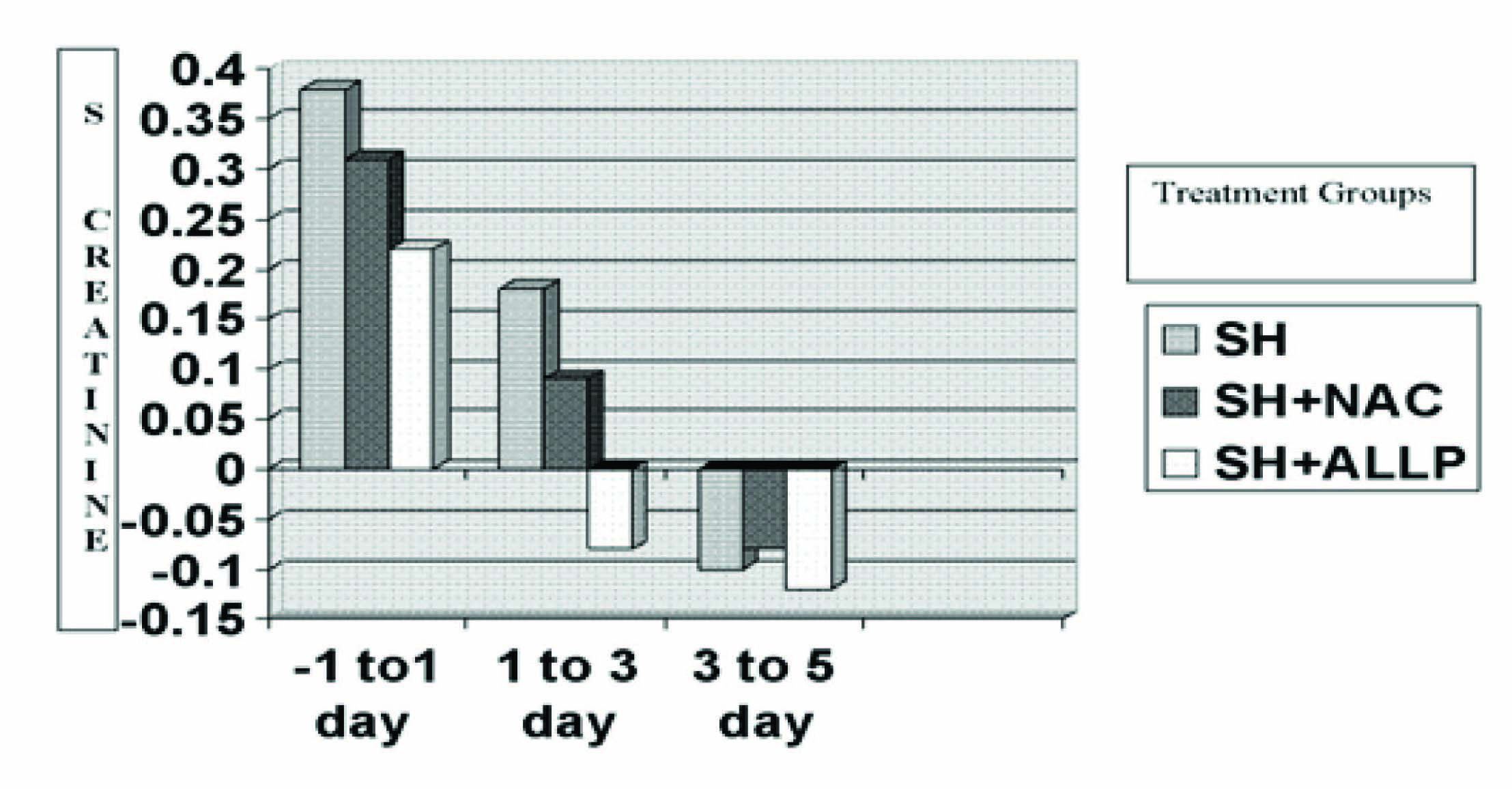
Rise in Scr in group 2 & 3 was significantly less than group 1 when compared with baseline levels at day 1 (p < .05). Fall in Scr levels was more significantly observed in group 3 (p < .001) than in group 2 (p< 0.01). Rise in blood urea levels in group 2 & 3 was less than in group1 compared to baseline levels (p <0.05). A fall in blood urea when compared to control group 1 was highly significant in group 3 from the 1st to 5th day (p < .001) than in group 2 (p < 0.01) [Table/Fig-6a and 6b]. There is a significant difference in the levels of Scr and blood urea between the treatment groups as well as between controls versus treatment (p< 0.0001).
Change in the levels of serum creatinine(mg/dl) in the three treatment groups in patients receiving visipaque dye
Change in the levels of blood urea (mg/dl) in the three treatment groups in patients receiving visipaque dye
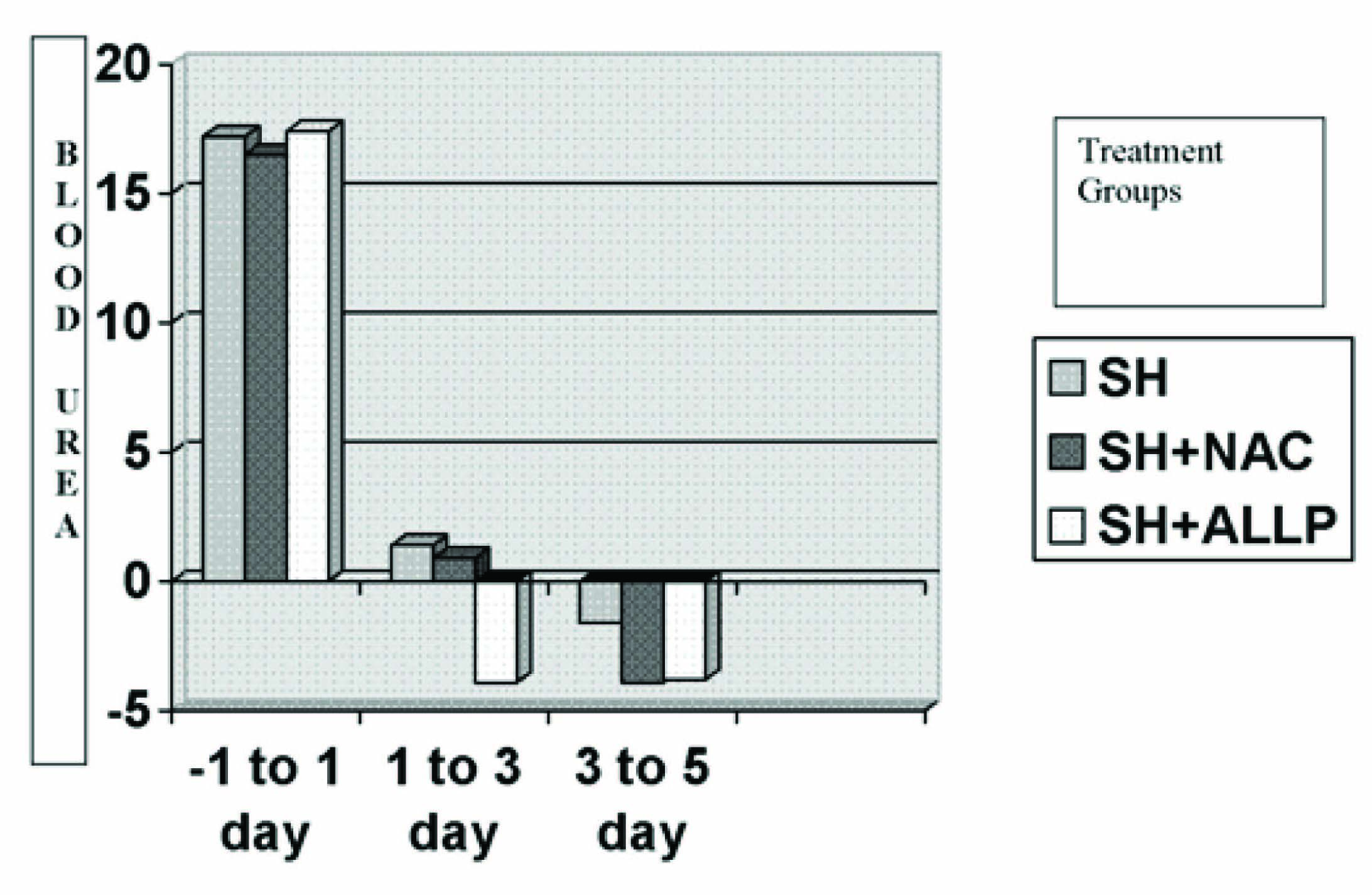
Secondary Endpoints
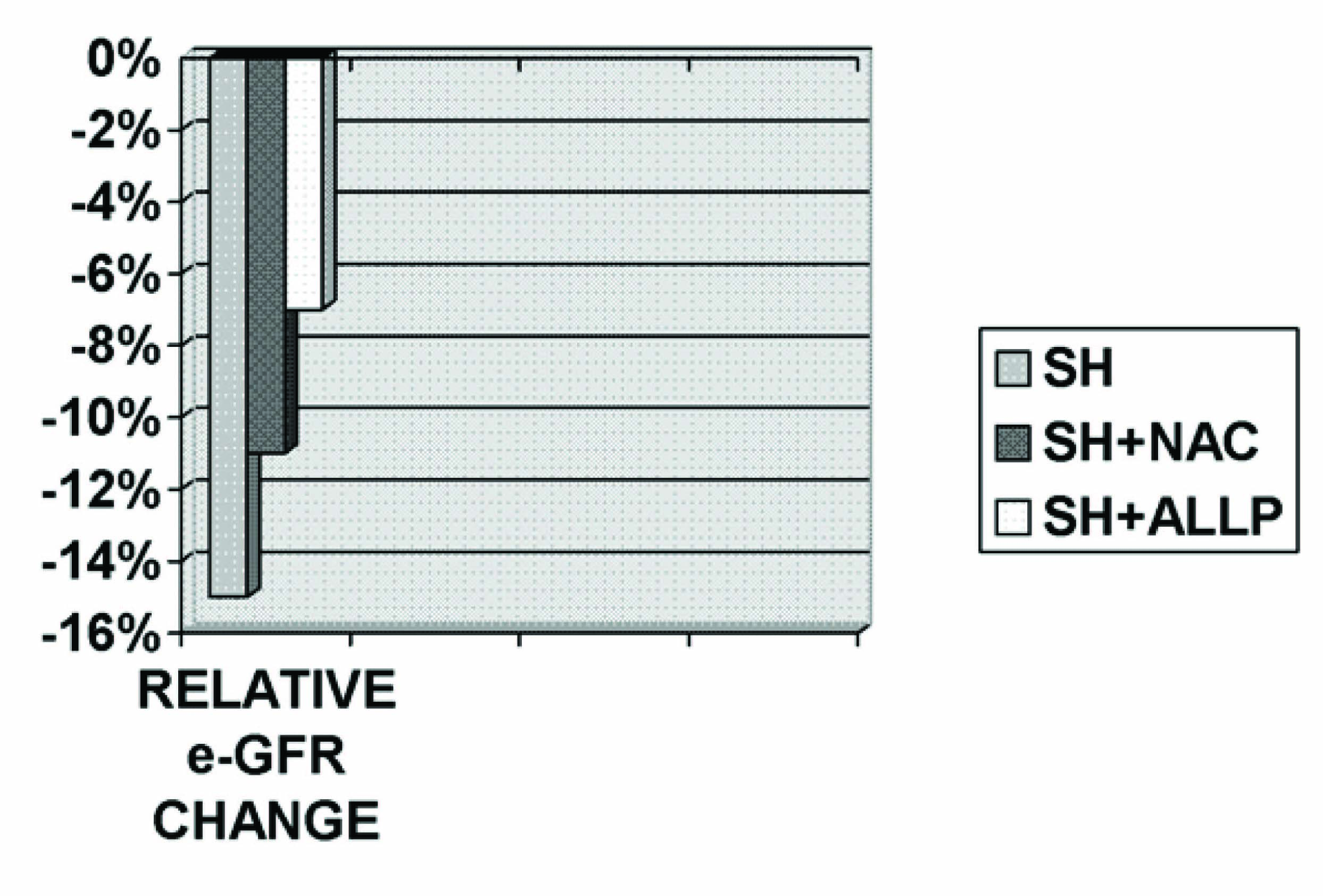
Overall, the e-GFR decreased from a median 72 (interquartile range 65 to 81) ml/min/1.73 m2 (p <0.001). The decrease in e-GFR was more prominent in control saline group when compared to SH plus NAC (p<0.01) and SH plus Allopurinol (p < 0.001) [Table/Fig-7].
The median decrease in e-GFR was 15% in saline control versus 11% and 7% in group 2(SH+NAC) and group 3(SH+ALLP) respectively
Discussion
Renal dysfunction is a widely recognised complication of cardiac catheterisation and percutaneous coronary interventions [14]. Several authors have published in-depth review articles, most notably Katzberg [15] and Maeder et al., [16] who performed a thorough review of urologic contrast agents and their potential effects. Although the exact mechanisms of CIN have yet to be fully elucidated, several causes have been described. Increased adenosine, endothelin, and free radical–induced vasoconstriction and reduced nitric oxide and prostaglandin-induced vasodilatation have been reported. Oxygen free radicals are produced during intra-renal adenosine catabolism to xanthine. These mechanisms cause ischemia in the deeper portion of the outer medulla, an area with high oxygen requirements and remote from the vasa recta supplying the renal medulla with blood. Contrast agents also have direct toxic effects on renal tubular cells causing vacuolization, altered mitochondrial function and apoptosis [17]. Although many of their concepts still hold true, we intended to concentrate on risk-factor analysis and current pathophysiological aspects of CIN.
Elevated serum uric acid levels are associated with hypertension, metabolic syndrome, chronic Kidney Disease and cardiovascular disease [18]. Investigating the potential role of urate reduction in the cardio renal disease, especially using urate-lowering agents is currently under way. More recently, experimental studies have suggested that uric acid contributes to these conditions by stimulating the renin–angiotensin system and reducing bioavailable levels of endothelial nitric oxide, resulting in renal vasoconstriction and possibly increasing blood pressure. Cell culture studies have documented that soluble uric acid has numerous acute proinflammatory and vasoconstrictive effects independent of intrarenal crystal deposition. For example, soluble uric acid can inhibit endothelial cell proliferation and migration as well as inhibit endothelial nitric oxide bioavailability [19–21]. Soluble uric acid can also induce neutrophil and monocyte chemotaxis [22]. Soluble uric acid can also activate proximal tubular cells in culture, resulting in stimulation of p38 MAP kinases and NF-αB, resulting in an inhibition of cell proliferation and the release of MCP-1 [23,24]. The stimulation of NOX-dependent ROS results in the activation of the MAPK kinases, p38 and Erk that can stimulate inflammatory and proliferative effects [23]. The major novel finding was that modest hyperuricemia markedly exacerbated renal progression. Specifically, hyperuricemic RK+OA rats showed more renal hypertrophy, hypertension, proteinuria, impaired renal function, greater glomerulosclerosis and interstitial fibrosis [25]. An interesting observation was that allopurinol was markedly effective at lowering uric acid and preventing OA-induced renal dysfunction, proteinuria, hypertension, vascular disease, renal hypertrophy, and renal scarring [9]. Epidemiological studies have revealed that uric acid concentrations predict the progression of chronic kidney disease [9]. Large epidemiologic studies have revealed an independent predictive role for uric acid in renal disease. Furthermore, in a recent study of 6400 subjects with normal renal function, a serum uric acid of >8.0 mg/dl, when compared with a serum uric acid level of <5.0 mg/dl, was associated with a 2.9-fold increased risk for developing renal insufficiency within 2 year in men and a 10.0-fold increased risk in women . This increased relative risk was independent of age, body mass index, systolic BP, total cholesterol, serum albumin, glucose, smoking, alcohol use, exercise habits, proteinuria, and hematuria. Indeed, an elevated uric acid was more predictive for the development of renal insufficiency than proteinuria [26].
The main finding of our study was that Allopurinol is superior to n-Acetylcysteine alone or with saline hydration in preventing CIN.This effect of allopurinol is consistent with the finding of a previous study [27]. The benefit of allopurinol correlated with its ability to lower uric acid. More importantly, it is possible that some of the benefit may also relate to the known ability of allopurinol to prevent oxidant formation, its anti-inflammatory, secondary proliferative pathway modulating effects which may be comparable to the pleitropic effects of statins [28–30].
Conclusion
Contrast induced nephropathy post cardiac interventions has become a major problem of morbidity and mortality which is rising in parallel to the increase in the number of cardiovascular events. From the vast arena of preventive measures currently available none has proved to be completely effective. Serum uric acid recently emerging as a biomarker for cardio-renal problems has paved the pathway for studying the effects of allopurinol a uric acid synthesis inhibitor in preventing CIN in a better way than the standard n-acetylcysteine most notably because of its pleiotropic effects. So, our study provides more consolidated data on the benefits of using allopurinol as a preventive measure for CIN as the current data available is scarce.