Case Report
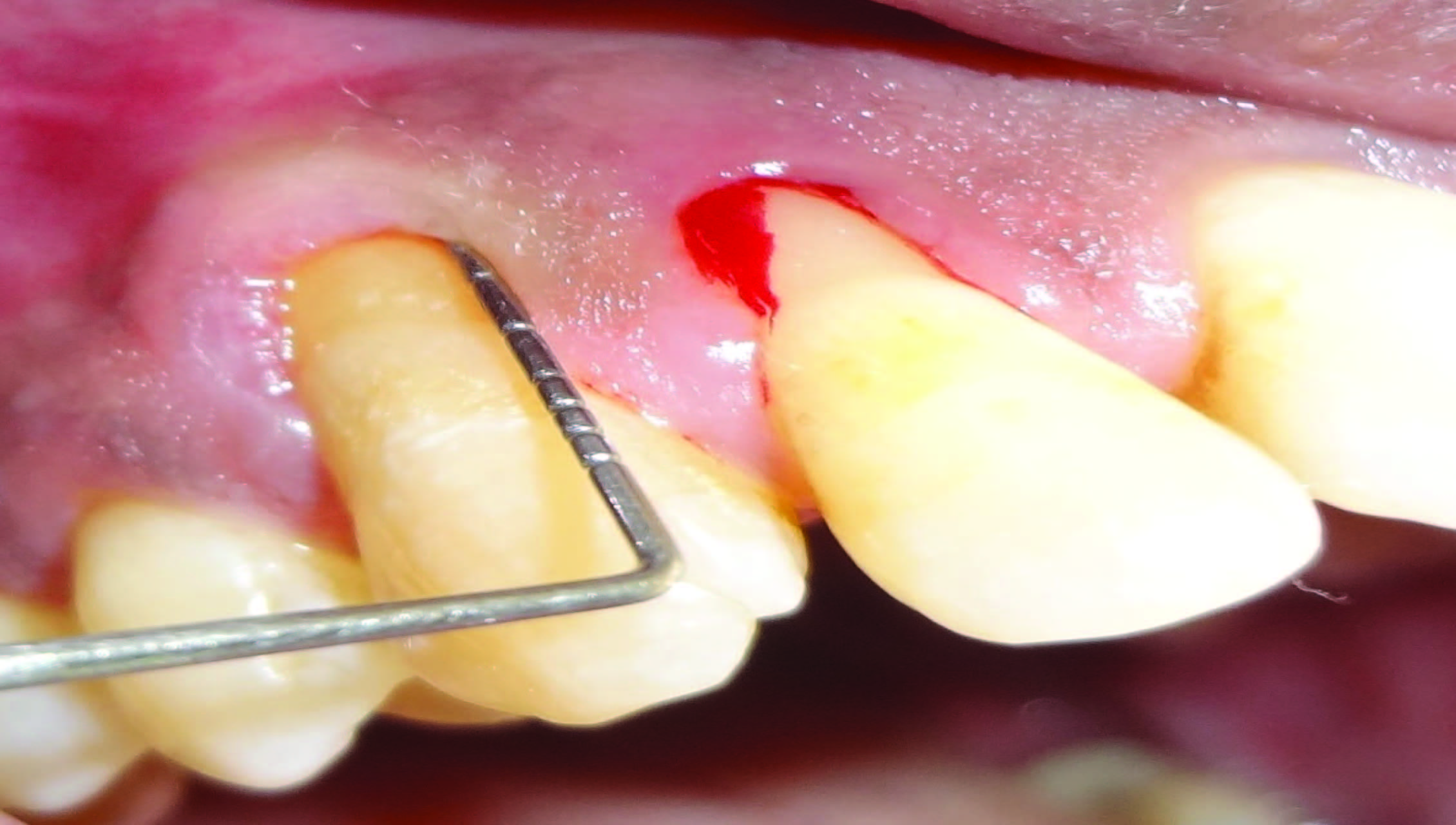
A 45-year-old systemically healthy male patient reported to the Department of Periodontology, Rajarajeswari Dental College and Hospital, Bangalore, India, with a chief complaint of sensitivity and pus discharge from maxillary right canine. Clinical examination revealed gingival abscess with maxillary right canine (#13) and Millers class II recession affecting maxillary right lateral incisor (#12) and canine (#13) with a clinical attachment loss (CAL) of 12 mm and 7 mm respectively [Table/Fig-1]. In addition there was a probing pocket depth (PPD) of 7 mm and 9 mm on distal and mesial aspect of #12 and 5 mm on distal aspect of #13. Moreover, both the teeth had extensive bone resorption as confirmed by radiographic examination [Table/Fig-2]. Fremitus test was found to be negative. The case was referred to the Department of Conservative Dentistry & Endodontics where vitality of #13 and 12 were checked and found to be vital.
Pre-operative view of recession defect. (Note: Bleeding seen is due to recording of clinical measurements.)

Presurgical therapy
Based on the findings, abscess drainage in relation #13 and routine full mouth scaling was performed, followed by root debridement in relation #14-11. The patient was prescribed antibiotics for a period of 5 d and was recalled after 4 wk for the surgery.
PRF preparation
Autologous tissue would be an ideal material of choice for soft-tissue augmentation if it could be obtained by a simple process with good predictability. Need for tissue harvesting, access incisions, postoperative recovery, and often unpredictable graft survival and longevity have encouraged surgeons to consider other available minimally invasive techniques and materials.
This prompted us to use L-PRF matrix for the regeneration of periodontal hard and soft tissues and assess its relevance clinically and radiologically.
PRF preparation requires a centrifuge and collection kit including a 24 gauge butterfly needle and 9 ml blood collection tubes. After obtaining the informed consent, whole blood is drawn, into the tubes without anticoagulant and is immediately centrifuged at 3000 rpm for 10 min [1]. The outcome is a fibrin clot containing platelets in the middle of the tube, between the red blood cell layer at the bottom and acellular plasma at the top [Table/Fig-3]. This clot is removed from the tube and the attached red blood cells scraped off and discarded. The PRF clot is then placed between two gauzes and pressed thereby squeezing out the fluids in the fibrin clot to obtain a matrix/membrane.
PRF clot after centrifugation

Surgical procedure
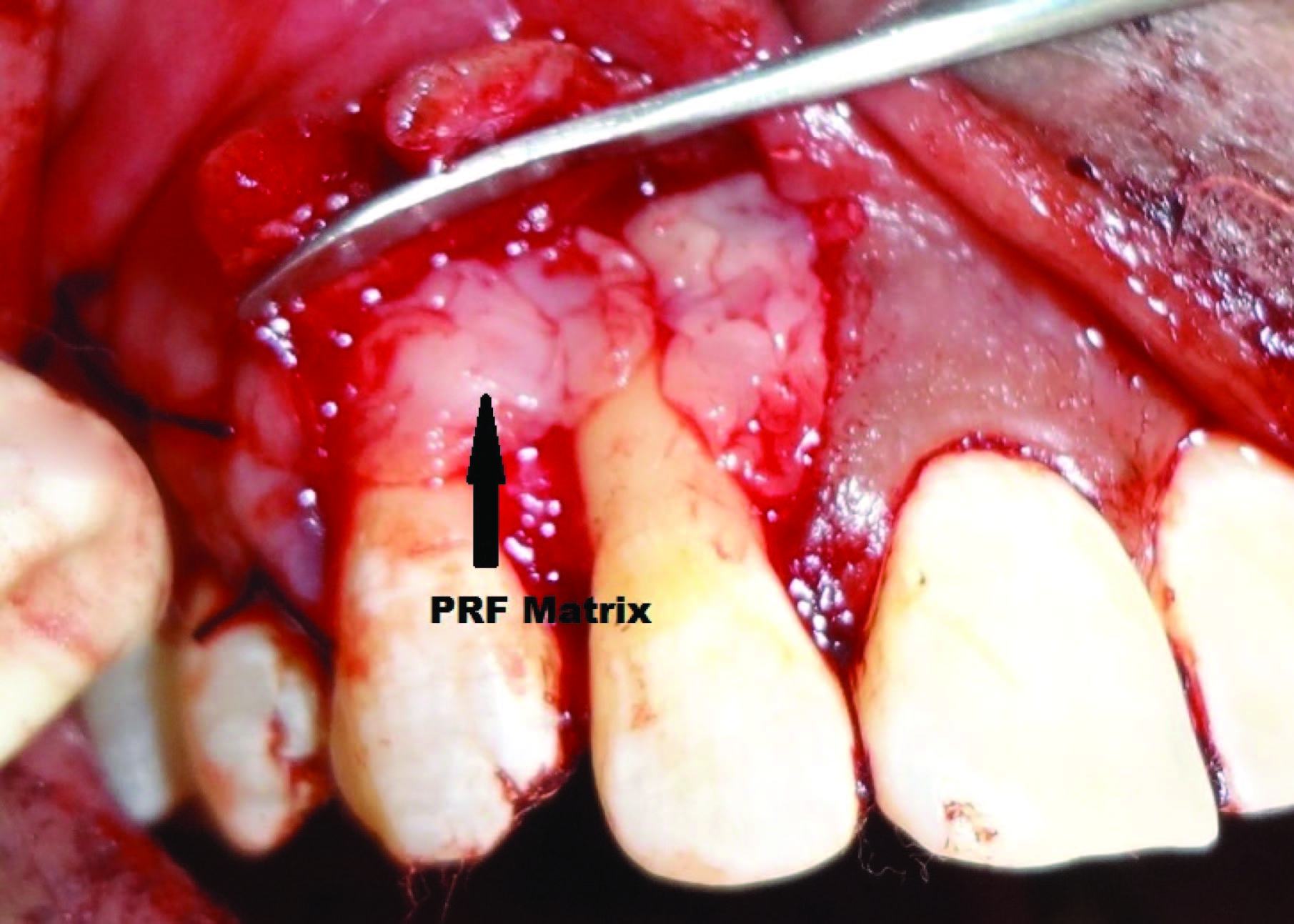
A coronally advanced flap along with bone regeneration was planned for root coverage as well as to treat the bony defect in relation to #12 and 13. After administering adequate local anaesthesia, two oblique, divergent bevelled incisions extending beyond the mucogingival junction were made at the mesial and distal line angles of the most mesial and the most distal teeth with gingival recession. Intrasulcular incisions were made along the margins of # 12 &13 allowing for a full thickness flap to be raised. A split thickness flap was raised from the mucogingival junction to allow coronal repositioning of the flap without tension. All papillae were deepithelialized to create a connective tissue bed. After elevation of the flap, a thorough debridement was done. A vertical osseous defect was observed on the mesial surface of root of #12 [Table/Fig-4]. Bioactive synthetic bone graft particulate (Perioglas®, FL, USA) was condensed into the defect. The prepared L-PRFmatrix was then adapted over the bone graft placed as well as the exposed root surface of #12 & 13 [Table/Fig-5]. The flaps were then coronally advanced and sutured with interrupted and sling sutures. Stabilization of the blood clot was achieved by the application of gentle pressure for 3 min. The surgical site was protected with a tin foil and periodontal dressing.
Intrabony defect after debridement

PRF membrane placed over the bone graft and exposed root surface
Post-operative care
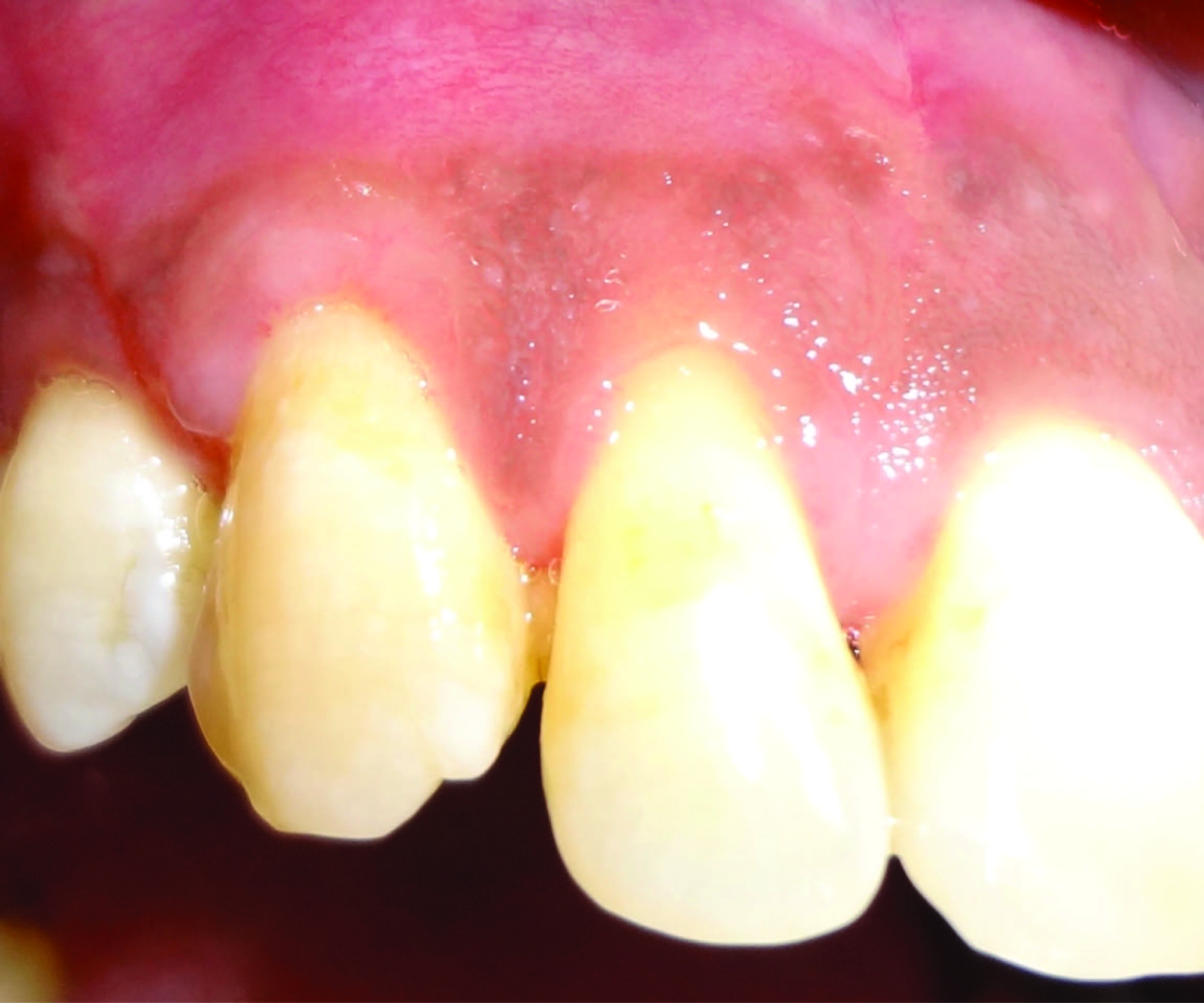
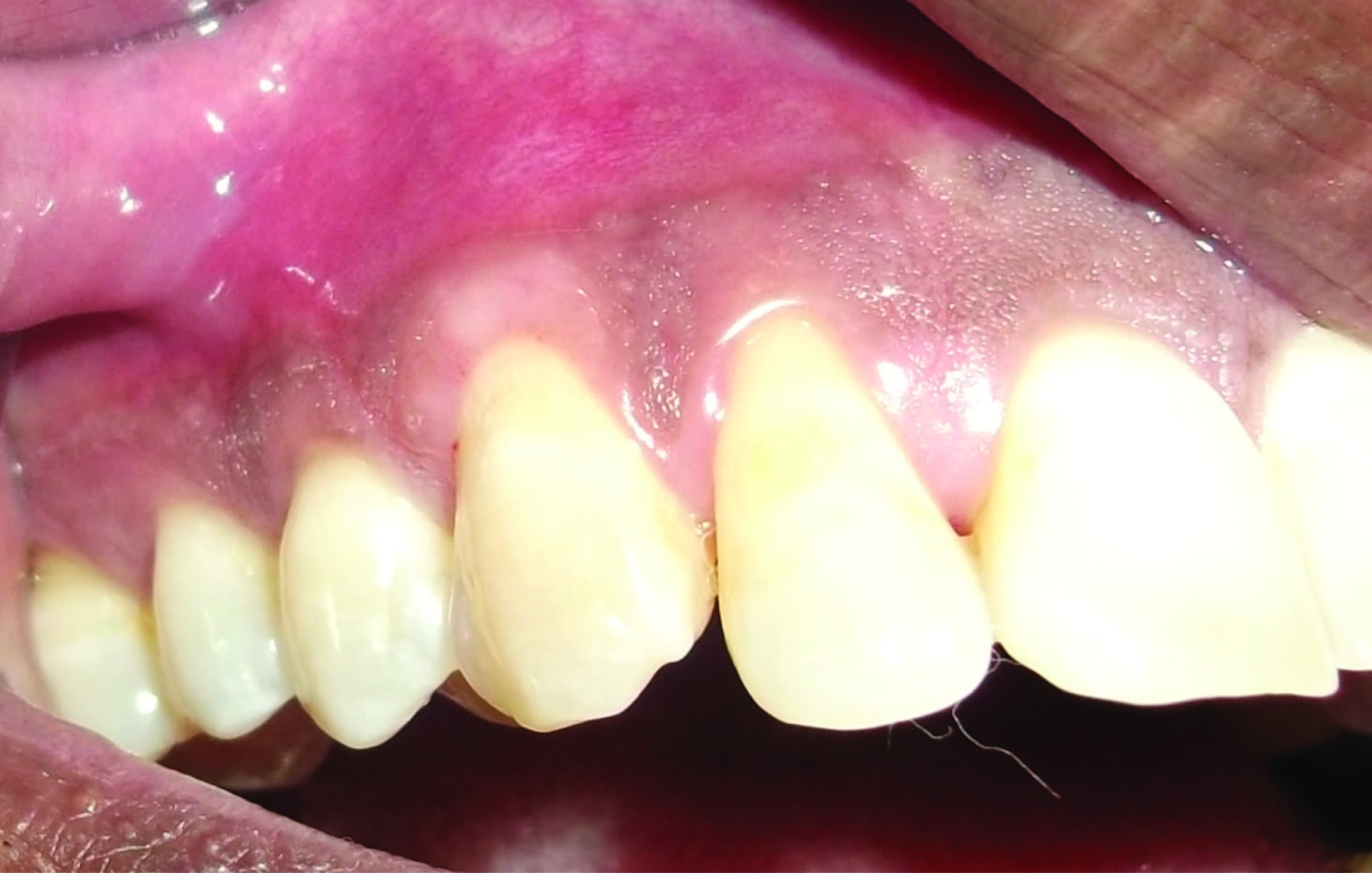
A protocol for the control of pain and bacterial contamination consisting of analgesics (Combiflam, thrice daily for 3 d) and antibiotics (Amoxicillin 500mg, thrice daily for 5 d) was prescribed. Patient was advised not to brush his teeth in the operated area until after suture removal two weeks later. He was instructed to rinse the mouth with a 0.2% chlorhexidine solution, twice a day for one minute, for three weeks. Fifteen days after surgical treatment, the patient was reviewed and instructed in mechanical tooth cleaning in the operated area using a soft toothbrush and Charters technique. The patient was recalled for prophylaxis one month after suture removal and at 3 and 6 months [Table/Fig-6,7].
Post operative clinical view at 3 months
Post operative clinical view at 6 months showing gain in attachment
Findings at clinical and radiographical review
Re-examination at six months after the periodontal surgery revealed reduction in PPD from 7 mm to 2 mm (distal) and 9 mm to 3 mm (mesial) on #12 & 5 mm to 2 mm (distal) on #13 respectively as well as gain in clinical attachment level of 9 mm and 5 mm irt #12 & 13 respectively. Significant radiographic bone formation was observed in the periodontal intrabony defect at six months [Table/Fig-8].
Post operative radiograph at 6 months showing defect fill (Yellow triangle)

Discussion
The present case report evaluated the clinical efficacy of L-PRF matrix in the treatment of gingival recession in association with a hard tissue defect. The grafted sites displayed rapid clinical healing, no flap reopening, and complete coverage of root with excellent tissue contour, colour and excellent bone density.
In our case, a significant reduction in PPD and CAL gain was found. Significant radiographic bone formation in the periodontal intrabony defect was also observed supporting the role of various growth factors present in the L-PRF matrix in accelerating the soft and hard tissue healing. A high degree of gingival maturation was observed after healing with a thickening of keratinized gingival tissues that improved the aesthetic integration. In addition, the use of L-PRF matrix seemed to reduce postoperative pain and oedema, and limited even minor infectious phenomena as observed by Toffler et al., [2]. Further, the elasticity and strength of the matrix made it more amenable to suture (Corso MD et al.,) [3].
Our result was in accordance with that of Anilkumar et al., [4] who reported complete root coverage with excellent gingival tissue status after six months, where PRF membrane along with laterally displaced flap was used for the treatment of an isolated recession defect.
Chang et al., [5] observed that PRF application exhibited pocket reduction and gain in clinical attachment along with increased post-operative radiographic density in the treated defects.
Pradeep and Sharma [6] also found greater reduction in probing depth, greater gain in periodontal attachment level and greater bone fill in 3-wall intrabony defects treated with PRF and open flap debridement (OFD) when compared to OFD alone.
Mechanism of Action
The L-PRF preparation process creates a gel like fibrin matrix, which incorporates platelets, leukocyte, cytokines, and circulating stem cells. PRF progressively releases cytokines over a period of time (7-11d), during fibrin matrix remodelling [7].
Chang et al., reported that PRF activation brings about expression of phosphorylated extracellular signal-regulated protein kinase (p-ERK) and osteoprotegrin (OPG) signifying its benefits for bone regeneration. Light and scanning electron microscopy showed leucocytes proliferating and interacting with osteoblasts leading to initiation in mineralization process [8]. Moreover, L-PRF is capable of increasing osteoblast attachment, proliferation and simultaneously up-regulating collagen-related protein production all of which would effectively promote bone regeneration.
The matrix acts like a fibrin bandage that accelerates the healing of wound edges. It also provides a significant postoperative protection of the surgical site and seems to hasten the integration and remodelling of the grafted biomaterial (Kumar et al.,) [1].
Advantages
Apart from obtaining the matrix by compressing the clot between two sterile gauze, the protocol is very simple, and many PRF clots can be produced in <20 minutes. Each PRF membrane concentrates most platelets and more than half of live and functional leukocytes from a 9-ml blood harvest [9] which releases high amounts of growth factors (such as transforming growth factor-β1 [TGFβ-1], platelet-derived growth factor-AB [PDGF-AB], vascular endothelial growth factor [VEGF]), and matrix glycoprotiens (such as thrombospondin-1) during a period of seven days in vitro [9].
They also have advantages like less surgical time, elimination of second surgical site and potential healing difficulties associated with membranes and less resorption during healing.
He et al., found PRF to be superior to PRP, in the expression of alkaline phosphatase (ALP) and induction of mineralization. They opined that PRF released autologous growth factors gradually and expressed stronger and more durable effect on proliferation and differentiation of rat osteoblasts than PRP in vitro [10].
When compared to PRP, L-PRF has the characteristic of polymerizing naturally and slowly during centrifugation. Further, the thrombin concentrations acting on the collected autologous fibrinogen are almost physiologic because there is no bovine thrombin addition. Weak thrombin concentrations imply a very significant percentage of equilateral junctions. These connected junctions allow the establishment of a fine and flexible fibrin network able to support cytokines enmeshment and cellular migration. Moreover, this 3-dimensional organization will give great elasticity to the fibrin matrix thus obtaining a flexible, elastic, and very strong PRF membrane [11].
Disadvantage
The success of this technique entirely depends on the speed of blood collection and transfer to the centrifuge. If the duration required to collect blood and launch centrifugation is overly long, failure will occur: The fibrin will polymerize in a diffuse way in the tube and only a small blood clot without consistency will be obtained [11].
Conclusion
The present case report substantiates the use of L-PRF matrix in the treatment of bony defect and recession. The ease of applying the matrix and its beneficial outcomes holds promise for further well designed and properly controlled clinical trials. Further histologic studies may provide evidence for L- PRF matrix’s capacity for and impact on wound healing, soft and hard tissue reconstruction and augmentation procedures, especially in periodontal therapy.
[1]. Kumar VR, Shubhashini N, Platelet rich fibrin: a new paradigm in periodontal regenerationCell Tissue Bank 2013 14(3):453-63. [Google Scholar]
[2]. Toffler M, Toscano N, Holtzclaw D, Corso MD, Dohan DM, Introducing Choukroun’s platelet rich fibrin (PRF) to the reconstructive surgery milieuJ Implant Adv Clin Dent 2009 1:22-31. [Google Scholar]
[3]. Corso MD, Toffler M, Dohan DM, Use of an autologous leukocyte and platelet-rich fibrin (L-PRF) membrane in post-avulsion sites: an overview of Choukroun’s PRFJ Implant Adv Clin Dent 2010 1:27-35. [Google Scholar]
[4]. Anilkumar K, Geetha A, Umasudhakar TR, Ramakrishnan T, Vijayalakshmi R, Pameela E, Platelet-rich-fibrin: a novel root coverage approachJ Indian Soc Periodontol 2009 13:50-54. [Google Scholar]
[5]. Chang Y-C, Wu K-C, Zhao J-H, Clinical application of platelet-rich fibrin as the sole grafting material in periodontal intrabony defectsJ Dent Sci 2011 6:181-88. [Google Scholar]
[6]. Pradeep AR, Sharma A, Treatment of 3-wall intrabony defects in chronic periodontitis subjects with autologous platelet rich fibrin—a randomized controlled clinical trialJ Periodontol 2011 82(12):1705-12. [Google Scholar]
[7]. Dohan DM, Choukroun J, Diss A, Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part II: Platelet-related biologic featuresOral Surg Oral Med Oral Pathol Oral Radiol Endod 2006 101:e45-50. [Google Scholar]
[8]. Chang I-C, Tsai C-H, Chang Y-C, Platelet-rich fibrin modulates the expression of extracellular signal-regulated protein kinase and osteoprotegerin in human osteoblastsJ Biomed Mater Res 2010 95A:327-32. [Google Scholar]
[9]. Mazor Z, Horowitz RA, Corso MD, Prasad HS, Rohrer MD, Dohan Ehrenfesti DM, Sinus floor augmentation with simultaneous implant placement using choukroun’s platelet-rich fibrin as the sole grafting material: A radiologic and histologic study at 6 monthsJ Periodontol 2009 80(12):2056-64. [Google Scholar]
[10]. He L, Lin Y, Hu X, Zhang Y, Wu H, A comparative study of platelet-rich fibrin (PRF) and platelet rich plasma (PRP) on the effect of proliferation and differentiation of rat osteoblasts in vitroOral Surg Oral Med Oral Pathol Oral Radiol Endod 2009 108(5):707-13. [Google Scholar]
[11]. Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJ, Mouhyi J, Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part I: technological concepts and evolutionOral Surg Oral Med Oral Pathol Oral Radiol Endod 2006 101:e37-44. [Google Scholar]