Pap smear screening is still the primary modality in detecting premalignant lesions and carcinoma of cervix, which has resulted in marked reduction in incidence and mortality of cervical cancer by 85% [3].
The mortality related to cervical cancer can be substantially reduced through early detection and treatment. Therefore cytological evaluation still plays an important role for early detection of dysplasia or pre-invasive cervical carcinomas. However, errors from sampling, screening and interpretation, still concealed some unpleasant results [4].
The accuracy of the cytological diagnosis on pap smears mainly depends on the morphological features characteristic of dysplastic or malignant cells. Cytological changes caused by infections, drugs, hormonal fluctuations may closely resemble pre-malignant or malignant changes cytomorphologically [2]. In these cases colposcopy is required for differential diagnosis, which creates anxiety among patients [5]. When abnormal cells are detected, the precise categorization into premalignant or malignant is highly subjective.
To compare the nuclear morphometric parameters of premalignant and malignant lesions in cervico-vaginal pap smears.
Materials and Methods
Our study was a retrospective analysis of 60 cervical pap smears received in our department. Cases selected for our study had confirmed histopathological diagnosis. Bethesda system was used to categorize cervical smears into 3 groups.
Group I –LSIL (Low grade squamous intraepithelial lesion -20 cases)
Group II- HSIL (High grade squamous intraepithelial lesion -20 cases)
Group III –SCC (Squamous cell carcinoma –20 cases)
Image Analysis
We used a microscope with a 2.5x ocular and a 40x objective to visually select a field for analysis. A 640 x 400 pixel digital image of the field was produced by a camera on the microscope and frame grabber card in a PC. Images were stored in the computer memory. Around 25 nuclei per case were analysed using Image J 1.44C morphometric software for image processing and analysis (JAVA) developed by National Institute of Health (NIH), USA. The measurements were made on the cell images in a precise and reproducible manner, and stored for analysis.
Nuclear parameters analysed
Radius computed by averaging the length of radial line segments from the center of the nuclear mass to each of the points of the nuclear border.
Nuclear area was the area within the outlined nuclear perimeter.
Perimeter was measured as the distance around the nuclear border.
Diameter was the diameter of the circle with the same area as the outlined nucleus
Compactness of the cell nuclei calculated using the formula: perimeter2 / area [9].
The computer calculated the mean, standard deviation and range for all the nuclear features.
Inclusion criteria
Only LSIL, HSIL and Squamous cell carcinoma were considered for the study.
Exclusion criteria
ASCUS, AGUS and Pap smears which did not have confirmed histopathological diagnosis were excluded from the study.
Ethical clearance was obtained by the Institutional Ethics Committee.
Statistical Analysis
The results obtained by computerized cytomorphometry were compared between the three groups. Data were analysed to evaluate the most distinctive morphometric features of all the features available. The nuclear parameters between all the 3 groups were compared using ANOVA and between the groups using a post hoc test i.e., Bonferroni Multiple Comparisons Test. Statistical analysis was performed using statistical software Graph Pad In stat.
A p-value < 0.05 was considered as statistically significant.
Results
Our sample size included 60 cases with age ranging from 21-70 y. The age distribution of the cases is shown in [Table/Fig-1]. The clinical diagnoses included routine cervical cancer screening (50%), white discharge per vaginum (32%), cervical erosion (12%) and growth in the cervix (6%).
Age distribution of cases
| Age distribution | Group I = LSIL NO (%) | Group II = HSIL | Group III = SCC |
|---|
| 21- 30 | 3 (15%) | 1 (5%) | – |
| 31-40 | 6 (30%) | 3 (15%) | 3 (15%) |
| 41-50 | 5 (25%) | 9 (45%) | 4 (20%) |
| 51-60 | 3 (15%) | 6 (30%) | 7 (35%) |
| 61-70 | 3 (15%) | 1 (5%) | 6 (30%) |
| Total | 20(100%) | 20(100%) | 20(100%) |
The sample was categorized into 3 groups based on cytological features:
Group I –LSIL (Low grade squamous intraepithelial lesion -20 cases)
Group II- HSIL (High grade squamous intraepithelial lesion 20 cases)
Group III – SCC (Squamous cell carcinoma –20 cases)
Cytological features
LSIL (Low grade squamous intraepithelial lesion): Mild dysplasia (CIN I) – Squamous cells arranged in sheets or singly showing enlarged hyperchromatic nuclei, increased nuclear: cytoplasmic ratio. Few cells show slightly irregular nuclear membrane with distinct and well defined cell borders [10] [Table/Fig-2].
LSIL showing squamous cell arranged in sheets with mild dysplastic features. (Pap stain,X400)
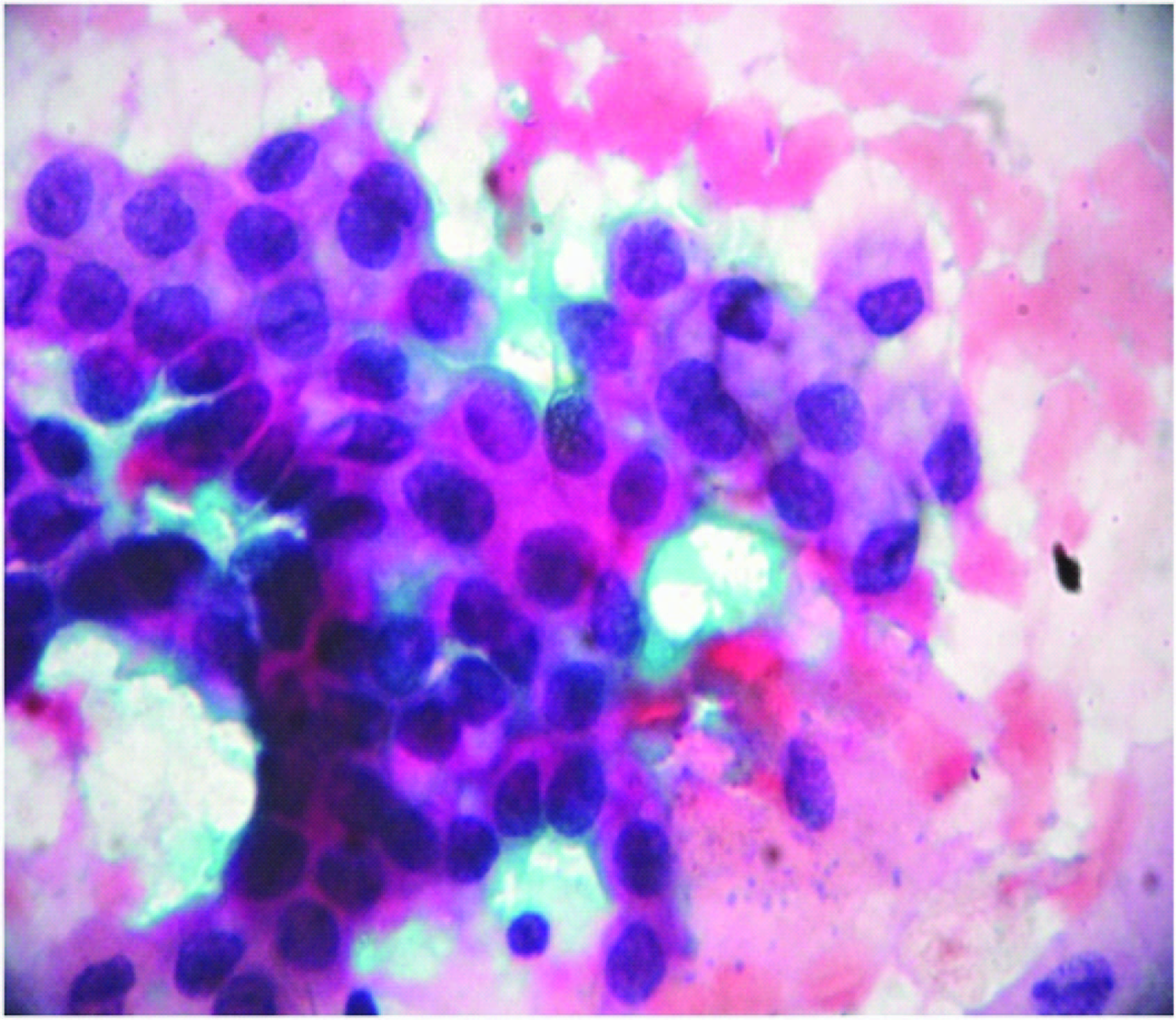
HSIL (High grade squamous intraepithelial lesion): Moderate and severe dysplasia (CIN II & III) – Squamous cells showing decreased cytoplasmic area with increased nuclear: cytoplasmic ratio. Cells with hyperchromatic nuclei with prominent nucleoli, irregular distribution of chromatin and irregular nuclear membrane [10] [Table/Fig-3].
HSIL showing squamous cells with increased nuclear:cytoplasmic ratio with moderate to severe dysplastic features. (Pap stain,X400)
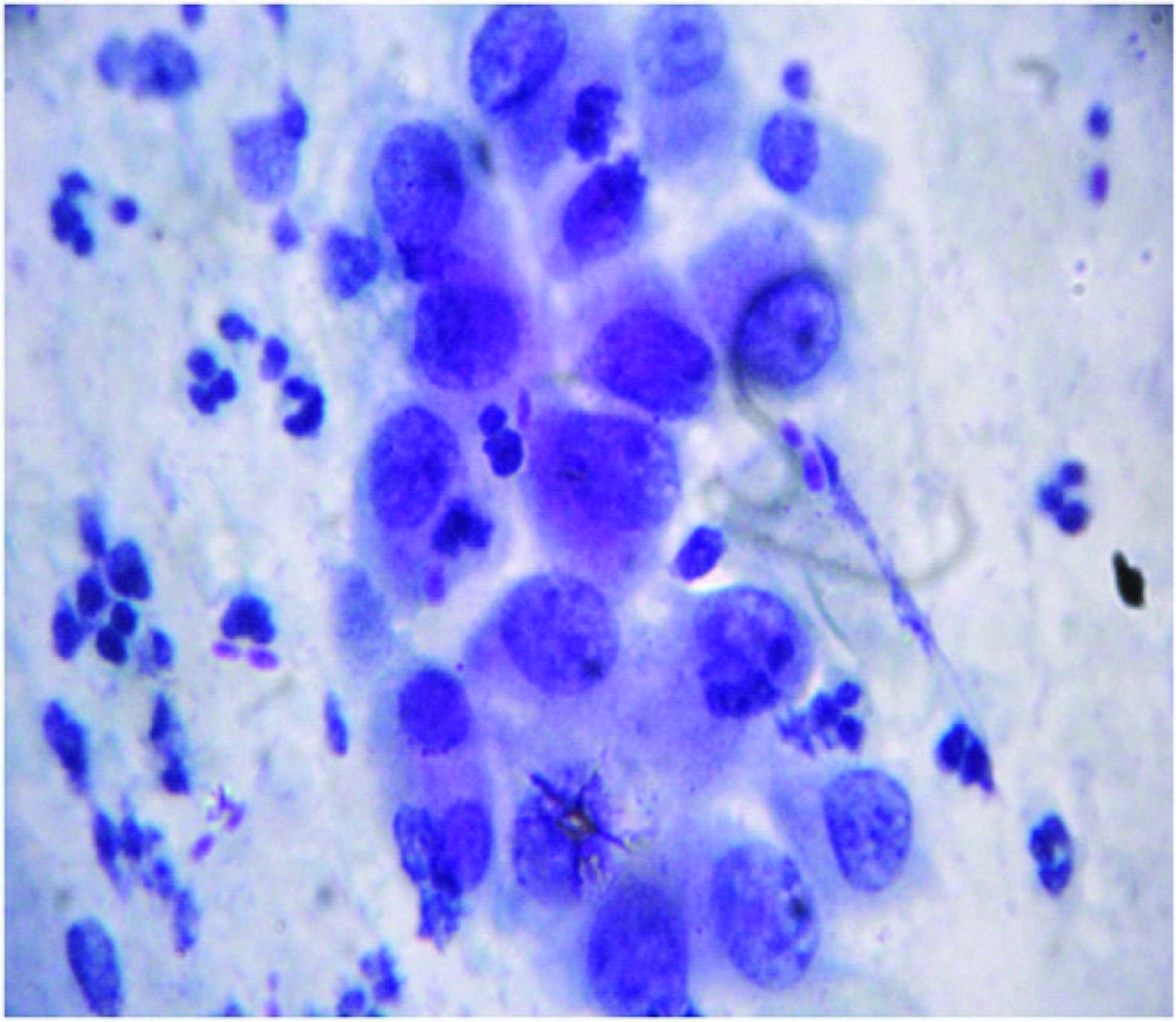
SCC (Squamous cell carcinoma): Malignant squamous cells are scattered in syncytial like aggregates, these cells shows features of HSIL in addition, they contain prominent macro nucleoli and marked irregular distribution of chromatin with or without keratinization and may be associated with tumor diathesis [10] [Table/Fig-4].
SCC showing squamous cells with features of HSIL with or without keratinization and may be associated with tumor diathesis. (Pap stain,X400)

LSIL were in the age group ranging from 31-40 y, HSIL were seen between 41- 50 y and SCC seen between 51-70 y.
Nuclear morphometric analysis was done using Image J 1.44C morphometric software for image processing and analysis [Table/Fig-5]. The basic results of our study are shown in [Table/Fig-6].
Nuclear morphometry image analysis representation

Nuclear morphometric analysis between the groups
| Nuclear features | LSIL (A) (n=20) Mean +/-SD (range) | HSIL(B) Disease (n=20) Mean +/-SD (range) | SCC(C) (n=20) Mean +/-SD (range) | Anova P value |
|---|
| Nuclear Area | 109.54 +/- 11.13 (98.6-120) | 132.7 +/- 17.31* (117-150) | 142.27 +/- 26.62 * (116-170) | <0.0001 |
| Perimeter | 24.69 +/-1.143 (23.6 – 25.9) | 27.12 +/- 1.49 + (25.4 - 29) | 28.18 +/- 2.72 + (25-30.9) | <0.0001 |
| Diameter | 7.72 +/- 0.45 (7.27-8.18) | 8.48 +/- 0.56 # (7.72 – 9.09) | 9.06 +/- 0.86 # $//(8.18 -9.9) | <0.0001 |
| Radius | 3.96 +/- 0.18 (3.7-4.1) | 4.31 +/- 0.29** (4.0-4.65) | 4.53 +/- 0.43 ** (4.0-4.8) | <0.0001 |
| Compactness | 5.54 +/- 0.045 (5.45- 5.63) | 5.56 +/- 0.068 (5.49-5.64) | 5.59 +/- 0.10 (5.59-5.63) | >0.05 |
* Significant difference in nuclear area of HSIL & SCC as compared to LSIL
+ Significant difference in perimeter of HSIL & SCC as compared to LSIL
# Significant difference in diameter of HSIL & SCC as compared to LSIL
$||significant difference in diameter of SCC as compared to HSIL
** Significant difference in radius of HSIL & SCC as compared to LSIL
Using one-way Analysis of Variance (ANOVA), the nuclear area, perimeter, diameter were found to be statistically significant (p<0.05). For comparisons between the individual groups, we employed post hoc test i.e. Bonferroni Multiple Comparisons Test. There was significant difference in nuclear area between LSIL (Group I) and HSIL (Group II) with a p-value (0.00001), LSIL (Group I) and SCC (Group III) with a p-value of (0.00001). There was significant difference in perimeter between LSIL (Group I) and HSIL (Group II) with a p-value (0.000001), LSIL (Group I) and SCC (Group III) with a p-value of (0.0000001) [Table/Fig-7].
| p-values |
|---|
| Anova | Group I -II | Group I-III | Group II-III |
|---|
| Nuclear area | 0.000005 | 0.00001 | 0.00001 | 0.186 |
| perimeter | 0.000001 | 0.000001 | 0.0000001 | 0.135 |
| diameter | 0.0000001 | 0.0000003 | 0.00003 | 0.016 |
| Radius range | 0.000002 | 0.00005 | 0.000003 | 0.067 |
| compactness | 0.123 | 0.280 | 0.056 | 0.287 |
Group I – Low grade squamous intraepithelial lesion (LSIL), Group II – High grade squamous intraepithelial lesion (HSIL), Group III – Squamous cell carcinoma (SCC)
There was significant difference in diameter between LSIL (Group I) and HSIL (Group II) with a p-value (0.0000003), LSIL (Group I) and SCC (Group III) with a p-value of (0.00003) and HSIL (Group II) and SCC (Group III) with a p-value (0.016). There was significant difference in radius between LSIL (Group I) and HSIL (Group II) with a p-value (0.00005), LSIL (Group I) and SCC (Group III) with a p-value of (0.000003).
There was no significant difference in nuclear area (0.186), perimeter (0.135), radius (0.067) between HSIL (Group II) and SCC with p value of (>0.05). There was no significant difference in compactness between all the three groups (Group I- II = 0.280, Group I-III = 0.056, Group II -III = 0.287) with p-value (>0.05) [Table/Fig-7].
The mean nuclear area, diameter and perimeter were useful in differentiating premalignant from malignant cervical smears. The squamous cell carcinoma cells showed higher values for nuclear area, perimeter, diameter when compared to LSIL and HSIL.
Discussion
Carcinoma cervix is the second most frequently occurring cancer amongst women globally [11]. An estimated 3,71,000 new cases of cervical cancers are identified every year and accounts for about 1,90,000 deaths annually. Developing countries like India account for 80% of these cases [12].
Pap smear screening is an essential part of a woman’s routine health care. It is a useful screening test to detect abnormal cells, including precancerous lesions, as well as malignant cancer cells. Both can be treated successfully if diagnosed at early stages. Routine cervical cancer screening has been shown to greatly reduce the number of new cervical cancers diagnosed each year and deaths from this disease [13].
A wide range of reactive, infectious and inflammatory conditions may give rise to cells which closely mimic that of pre-cancerous or malignant lesion which may lead to misdiagnosis, thus endangering patient lives. This may ultimately have a major impact on the management of disease [5,14]. The advantage of cytology in differentiating abnormal cells from normal cells has been widely recognized and accepted in cervical screening program. However, the false negative results may unnecessarily postpone the required treatment [5]. Hence, care should be emphasized while reporting Pap smears.
Despite well-established screening programs in US, nearly half of the cervical cancers come to light only in locally advanced stages. In developing countries like India, the disease is usually advanced at the time of diagnosis leading to increased mortality among women [1]. Hence, our study aimed to explore the possible role of nuclear morphometric analysis to improve the sensitivity and specificity for detection of pre-cancerous and cancerous conditions.
Cytological criteria for differentiating normal cells from abnormal cells are based on change in nuclear size, irregularity of nuclear shape and granularity of nuclear chromatin which will be assessed by cervical smears based on subjective criteria [4]. In contrast, in computed morphometry, the subjective criteria are turned into quantitative parameters [4]. The most widely used parameters in various malignancies include mean nuclear area, perimeter, diameter and N/ C ratio [15,16].
In our study, we had confirmed histopathological diagnosis for all selected cervical pap smears. LSIL – Mild dysplasia, HSIL – moderate to severe dysplasia and SCC – squamous cell carcinoma on histopathology.
In the present study the size related parameters (nuclear area, perimeter, and diameter) of the nucleus were appropriate parameters to differentiate between premalignant from malignant cervical smears. These parameters showed significant differences between LSIL, HSIL and squamous cell carcinoma which was highly significant with p-value < 0.0001.
Some of the breast studies have also measured long axis and short axis as nuclear morphometric parameters, but among the nuclear parameters nuclear area and perimeter are important [17–19].
In addition to these basic nuclear morphometric parameters, Murata et al., reported 27 morphological nuclear parameters which were categorized into more reasonable cytological features to achieve better clinical understanding of computed morphometric features [20].
In a study done by Huang et al., on cervical smears by PC based Cyto pathologic Image Analysis System and Support Vector Machine(SVM) showed that in dysplastic cells, the morphometric parameters like perimeter, nuclear area, maximum length, maximum width, N/C ratio were all found to be statistically significant with p-value of 0.001. These statistics showed that dysplastic cells have larger size (i.e. larger perimeter, area, maximum length, and maximum width), higher nuclear proportion (i.e. N/C ratio) [4].
Another study done by Nemec et al., used ploidy and chromatin pattern analysis as an aid for cervical smear diagnosis to analyse the morphology of Feulgen stained cell nuclei in cell populations. They showed efficient results to discriminate between normal and HSIL groups with 97% specificity and 88% sensitivity [5]. In a study done by Prasad et al., on exfoliated buccal mucosal cells in diabetes patients by cytomorphometric analysis showed that there was statistically significant increase in nuclear diameter in diabetic patients compared to controls [21].
In our study there was a gradual increase in nuclear area and perimeter in carcinoma when compared to premalignant lesions. The nuclear morphometric parameter which could significantly differentiate between LSIL and HSIL were nuclear area, perimeter, diameter and radius. These four parameters were useful to differentiate between LSIL and SCC which was statistically significant. Thus nuclear area, perimeter and diameter were highly significant in differentiating premalignant from malignant cervical smears with p-value of < 0.0001. Compactness was not statistically significant in differentiating between all the three groups with p-value >0.05 and thus there was no discrepancy noted in compactness of cell nuclei in any of the three groups.
Conclusion
Nuclear morphometry is thus a useful objective tool in the differentiating premalignant and malignant cervical smears. It is also helpful in diagnostic dilemmas which are encountered especially in gray zones on cervical smears, especially by ASCUS or AGUS. By combining the findings of clinical examination, cytomorphological and nuclear morphometric parameters, we can improve the diagnostic accuracy of cervical cancer screening and hence aid clinicians to apply appropriate treatment modalities.