Introduction: The odontogenic keratocyst (OKC) is a histopathologiocally and behaviourally unique and specific entity. It is the most aggressive and recurrent of all the cysts and shows characteristics resembling both cyst and a tumour. The unique nature of OKC and the recent shift of OKC as a tumour made us evaluate yet another factor, Inducible nitric oxide synthase an (iNos) enzyme which has been implicated in the tumourigenesis of various neoplasms.
Aims and Objects: The objective of the study was to analyse and compare the immunohistochemical expression of iNOS in odontogenic keratocysts (OKC’s) in variants of ameloblastoma affecting the oral cavity, to determine the neoplastic potential of OKC and to reinforce the classification of OKC as keratocystic odontogenic tumour.
Materials and Methods: Thirty two specimens, eight specimens each in OKC, follicular ameloblastoma, plexiform ameloblastoma and unicystic ameloblastoma, taken from the Oral Pathology Department were randomly selected for this study and were evaluated for epithelial expression of iNOS by immunohistochemistry
Results: Epithelial immunoreactivity to iNOS was strongly positive in 93.5% of follicular ameloblastomas, 68.7% of plexiform ameloblastomas, 66.9% of odontogenic keratocysts and 66.2% of unicystic ameloblastomas.
Conclusion: iNOS may be an important marker involved in the biological behaviour of OKC. Furthermore the presence of increased expression of iNOS in Follicular ameloblastomas followed by Plexiform ameloblastomas, OKCs and Unicystic ameloblastomas is yet another evidence to support that OKC could be considered as a neoplasm.
Introduction
Odontogenic keratocysts (OKCs) are of epithelial origin, first identified and described in 1876 and further characterized by Phillipsen in 1956 [1]. Pindborg and Hansen suggested the histologic criteria necessary to diagnose OKC in 1962. The cystic nature of odontogenic keratocyst (OKC) has long been debated, with some investigators classifying the OKC as a benign tumour. In recent years, the WHO has recommended that the term "keratocystic odontogenic tumour" (KCOT) replace the term "odontogenic keratocyst" (OKC), as it better reflects the neoplastic nature of the lesion [2]. Several factors, such as the cyst's aggressive behaviour, high mitotic activity histopathologically, and evidence of associated genetic and chromosomal abnormalities (eg, mutation of the PTCH gene) often seen in neoplasia, serve as the basis for this new classification [3].
The odontogenic keratocyst is a histopathologiocally and behaviourally unique and specific entity. It is the most aggressive and recurrent of all the cysts and shows characteristics, resembling both cyst and a tumour [4].
Ameloblastoma is a benign tumour that displays an insidious slow growth with a locally invasive behaviour and a high rate of recurrence. It is considered as the most clinically significant odontogenic tumour. Ameloblastomas and odontogenic keratocysts share many clinical features in common such as local aggressiveness, high recurrence rates [5].
This unique nature of OKC and the recent shift of OKC as a tumour made us evaluate yet another factor, Inducible nitric oxide synthase an enzyme which has been implicated in the tumourogenesis of various neoplasms.
Nitric oxide (NO) has been called a “double-edged sword” [6] with beneficial effects like -(antiviral, antiparasital, microbicidal, immunomodulatory and anti tumour effect) and deleterious effects like – (inhibition of enzyme functions, alteration of DNA, induction of lipid peroxidation, mutation of tumour suppressor genes, cytotoxicity, inhibition of mitochondrial respiration, depletion of antioxidant stores and hypoxia induced angiogenesis in cancer depending on the amount and conditions under which it is produced). In neoplastic tissue, NO is implicated as having a positive role in permitting tumour growth, including mutagenisity, angiogenesis and metastasis. NO is synthesized by a complex family of enzymes called nitric oxide synthases (NOS). The role of NO generated by iNOS is very complex. However, induced at the wrong place or at the wrong time iNOS has deleterious consequences and seems to be involved in the pathophysiology of different human diseases [7].
Benign neoplasms have been reported to show upregulation of iNOS expression. iNOS activity or expression has been examined in various human malignancies, and available evidence suggests that NO may play important role in tumour growth, progression or metastasis [8].
Expression of iNOS has been studied in various cysts and tumours and the reports have emphasized the significance of iNOS expression as an indicator of the malignant potential of cysts and tumours.
Aims and Objecives
To assess the immunohistochemical expression of iNOS in OKC's and the variants of ameloblastomas.
To understand the possible role of iNOS in the aggressiveness, biologic behaviour and neoplastic potential of OKC and variants of ameloblastoma.
Materials and Methods
A total of 32 specimens, eight specimens each in OKCs, follicular ameloblastoma, plexiform ameloblastoma and unicystic ameloblastoma, obtained from the Department of Oral Pathology, Vinayaka Missions Sankarachariyar Dental College, were randomly selected for this study. The sample size for this study was limited to available institutional archival cases. Orthokeratinised odontogenic keratocysts and recurrent ameloblastoma cases were excluded and Parakeratinised Odontogenic Keratocysts and Primary Ameloblastomas were included in the present study. Serial sections, 3 micro m thick, were taken from the tissue blocks and processed for immunohistochemical examination. The study was approved by the Research Ethical Committee of the Vinayaka missions University, Salem.
Immunohistochemical methods
The tissue sections were deparaffinized. Antigen retrieval was done by pressure cooker method using tris EDTA buffer solution. The endogenous peroxidase activity of the tissues was blocked by incubating them in 3% hydrogen peroxide for 10 min. The tissue sections were next incubated in 0.4% casein in phosphate buffered saline for 10 min to block the nonspecific binding sites. After this the tissue sections were incubated for one hour with the primary antibody (Rabbit polyclonal antibody to iNOS) Santa Cruz genetix. This primary antibody was diluted to a ratio of 1:50 in PBS. The slides were then incubated in post primary block and subsequently with HRP conjugated secondary antibody for 30 min each. Freshly prepared DAB – substrate working solution was added and incubated for five minutes. Finally after thorough washing in distilled water, the slides were counter stained with Mayer's hematoxylin, dehydrated in grades of alcohol and mounted with synthetic mounting media. A previously diagnosed pyogenic granuloma stained with iNOS was taken as positive control and a tissue from the present study, without staining with primary antibody, was taken as the negative control. Positive iNOS expression is seen as light brown granular stain.
Results
In the present study we observed that the iNOS synthase was expressed in the nucleus and cytoplasm of the lining epithelium of OKCs and in the peripheral tall columnar cells of Follicular, Plexiform, and Unicystic ameloblastomas [Table/Fig-1,2,3,4].
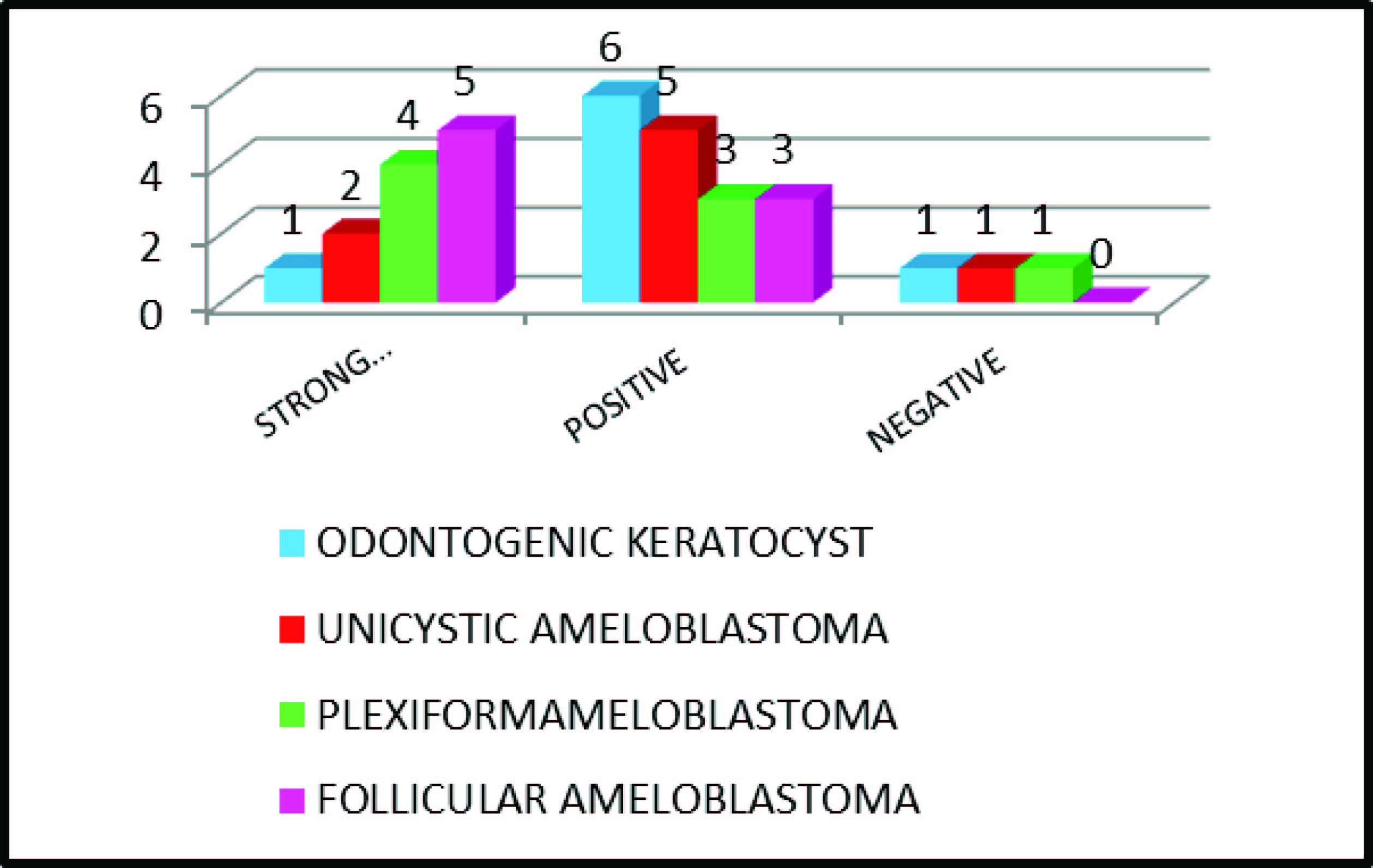
The proportion of positively staining cells and the strength of staining were combined to produce a semiquantitative scoring standard for immunohistochemistry. Nuclear and Cytoplasmic stains for iNOS were scored according to the presence and intensity of staining on the section, where (-) = unstained or Negative, (+) = yellowish brown or Positive, and (++) = dark brown or Strong Positive [Table/Fig-5].
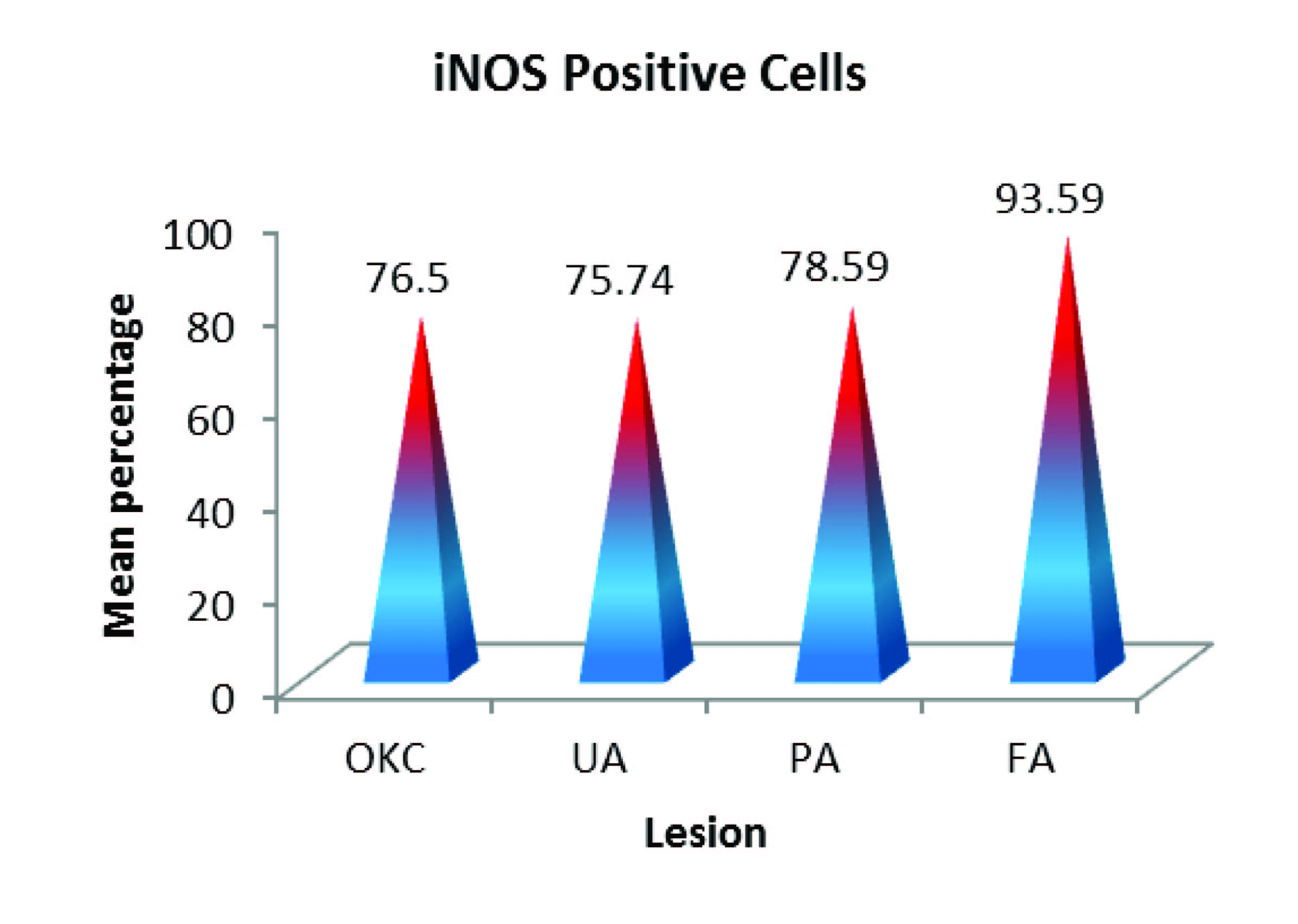
Additionally, on counting the number of positively stained cells for iNOS with the total number of tumour cells in three hot spot fields we observed that the average percentage of positive cells for iNOS was low in OKC (66.9%) , Plexiform (68.7%) and Unicystic ameloblastomas (66.2%) when compared to follicular ameloblastoma (93.5%) which was high [Table/Fig-6].
Statistical Analysis
Statistical analysis was performed using Analysis of variance test (ANOVA) after testing the confirmatory test of Normality by using P-P Plots and Tukey’s tests. p-value of less than 0.01 was considered as statistical significance. The percentage of positive cells for all the four groups is presented as mean and standard deviation (SD). The F statistics was calculated as the ratio of the variances. The p-value was less than 0.01, this showed that there is highly significant difference in the mean scores of the four groups with respect to the intensity percentage of iNOS Positive Cells [Table/Fig-7,8].
Once we had determined that differences exist among the means of the four groups, the post hoc range tests (Tukey’s tests) was done to determine which means differ and to identify the homogeneous subsets of means that are not different from each other. The results showed that the groups Odontogenic Keraticyst, Unicystic Ameloblastoma and Plexiform Ameloblastoma did not differ significantly with respect to the percentage of iNOS Positive Cells. But, these three groups significantly differed from FA [Table/Fig-9,10].
Discussion
In the literature, in cases of odontogenic cysts - the expression of iNOS has been studied only in RCs and in OKC, DC and RCs comparing with p53 protein expression. In case of tumours, the expression of iNOS was studied in tooth germ, benign and malignant ameloblastomas in order to prove the role of iNOS in tumourogenesis [9]. Secondly iNOS and VEGF expression was compared between primary, recurrent and malignant ameloblastomas using OKC as the control group in order to examine the relationship of this expression to angiogenesis and the clinical and biological behaviour of the tumour [10].
There are no articles in the literature comparing the expression of iNOS in odontogenic keratocyst with the three most common histological variants of ameloblastomas (follicular, plexiform and unicystic). Number of clinical papers, have reported that OKC behaves in every way as aggressive as a benign neoplasm such as an ameloblastoma [11]. These concepts led us to carry out this Pioneer study to reinforce once again that the OKCs do behave like a tumour, using the marker Inducible Nitric Oxide Synthase. A similar study with same group of samples like that of the present study (i.e. OKC, FA, PA & UA) was done by Noorieh et al., [12] to investigate and compare the expression of p53 and MDM2 proteins.
In the present study out of the eight samples in each lesion 7 OKCs [Table/Fig-1], 7 unicystic ameloblastoma [Table/Fig-3] and 7 plexiform ameloblastomas [Table/Fig-2] had positively stained for iNOS whereas all the eight samples of follicular ameloblastomas [Table/Fig-4] were positively stained and the p-value was 0.001(<0.01) indicating that the difference among each lesion was highly significant. This results of our present study was in contrast to the previous study by Wei-Liang Chen [10], where out of the 10 OKC samples iNOS was positively expressed in only one sample.
But, in case of ameloblastomas according to Kumamoto et al., [9] the peripheral tumour cells had + (weakly positive) and few keratinizing cells had ++ (moderately positive) iNOS expression. This result was similar to the present study where the iNOS expression was (+) in OKCs and unicystic ameloblastomas and (++) in plexiform and follicular ameloblastomas.
Additionally, scoring the percentage of positively stained tumour cells for iNOS with the total number of tumour cells in three hot spot fields gave us the quantative data of iNOS expression in the four lesions was almost similar, with a p-value 0.918 (>0.05 i.e. not significant) while the Follicular ameloblastomas had the highest percentage of positive cells, this may be due to expression of iNOS in both the peripheral odontogenic epithetlial cells and the central stellate reticulum like cells. The Plexiform ameloblastoma being a solid type of ameloblastoma showed less iNOS expression when compared to Follicular ameloblastomas, may be because of less stellate reticulum cell seen in the present cases hence only the peripheral cells were positive for iNOS.
The Unicystic ameloblastoma being a cystic variety of ameloblastoma had almost equal expression of iNOS when compared OKC and this results favoured the concept that OKCs behaved like ameloblastomas (histologically less aggressive variant) and the expression of Inducible nitric oxide synthase did contribute to the biological behaviour of these tumours. The results of the present study was in favour with the study done by Noorieh et al., [12] using the markers p53 and MDM2 Proteins in order to contribute to the similar biological behaviour of Odontogenic keratocyst and benign ameloblastomas .
High NO production in cyst and tumours may participate in bone resorption and cystic enlargement. This is because synthesis of matrix metalloproteinases (MMPs) can be activated by NO [13]. An increased expression of iNOS in OKC to the extent of the benign neoplasm (ameloblastomas) in our study and the already established findings of its role in cell proliferation, angiogenesis and production of proteolytic enzymes, suggests that there is definite role of iNOS in the development and progression of OKC. It also justifies the similar biological behaviour of the OKC and the less aggressive variants of ameloblastomas. This study establishes yet another evidence to support the notion of OKC being a neoplasm.
Recent studies of NOS activity and expression iNOS in solid tumours suggest, that NO may have a functional role in promoting solid tumour growth and progression. On the strength of this evidence, Thomsen LL et al., [14] reasoned that inhibition of iNOS, in tumours expressing the enzyme was required to fully confirm whether the role of NO was in promoting the growth and progression of cancer or as a host defense mechanism. Therefore, the expression of iNOS in odontogenic keratocysts and in variants of ameloblastomas reported in the present study and by other groups [14] suggests that an iNOS inhibitor may play an important role in the treatment of odontogenic lesions. Further studies are needed to prove this therapeutic approach.
Legal knowledge based on nursing qualification




Assessment of staining intensity of iNOS in Okc , Ua, Pa and Fa
| Lesion | Strong Positive (++) | Positive (+) | Negative (-) | Total number of cases |
| Odontogenic Keratocyst | 1 | 6 | 1 | 8 |
| Unicystic Ameloblastoma | 2 | 5 | 1 | 8 |
| Plexiform Ameloblastoma | 4 | 3 | 1 | 8 |
| Follicular Ameloblastoma | 5 | 3 | 0 | 8 |
Percentage of iNOS positive cells in okc , ua , papa and fa
| Lesions | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 | Case 8 | Average |
| OKC | 69% | 69.9% | 82.8% | 79.7% | 75.9% | 90.7% | 7.5% | - | 66.9% |
| UA | 75.4% | 72.3% | 72.6% | 71.7% | 69.1% | 81.4% | 87.7% | - | 66.2% |
| PA | 72.6% | 77.2% | 69.5% | 60% | 84.6% | 97.2% | 89% | - | 68.7% |
| FA | 90.2% | 90.1% | 86.5% | 97.1% | 93.8% | 99.6% | 97.8% | 93% | 93.5% |
Means, standard deviation (SD) and results of ANOVA test for the comparison between iNOS staining intensity in the four groups, ** - Highly significant
| Lesion | N | Mean | SD | F Statistics | P |
| OKC | 7 | 76.50 | 8.49 | 7.56 | 0.001** |
| UA | 7 | 75.74 | 6.55 |
| PA | 7 | 78.59 | 12.63 |
| FA | 7 | 93.51 | 4.48 |
| Total | 29 | 81.51 | 11.03 | | |
Expresion of iNOS variation for odontogenic cyst & variants of ameloblastoma
Tukey’s Test (Post hoc range test)
| iNOS Positive Cells |
| Tukey HSD |
| Lesion | N | Subset for alpha = .05 |
| 1 | 2 |
| UA | 7 | 75.74 | |
| OKC | 7 | 76.50 | |
| PA | 7 | 78.59 | |
| FA | 8 | | 93.51 |
| P | | 0.918 | 1.000 |
Tukey’s Test (Post hoc range test)
Conclusion
Both genetic and molecular research regarding OKCs has led to an increasing amount of knowledge and understanding of their physiopathological pathways. Markers known to be induced in response to growth factors, tumour promotors, cytokines, bacterial endotoxins, oncogenes, such as iNOS, may indeed shed new light on the biological mechanisms involved in the development of these benign but yet aggressive neoplasms of the jaws. Although, iNOS has rarely been used to assess the biological activity of the OKCs, the results portrayed in the present study suggest that iNOS may be an important marker involved in the biological behaviour of the OKC.
[1]. HP Philipsen, Om keratocystedr (Kolesteratomer) and kaeberneTandlaegebladet. 1956 60:963-71. [Google Scholar]
[2]. HP Philipsen, Keratocystic odontogenic tumour. In: Barnes L, Eveson JW, Reichart PA, Sidransky D, eds. World Health Organization Classification of Tumours: Pathology and Genetics Head and Neck Tumours. Lyon, FranceIARC Press 2005 :306-07. [Google Scholar]
[3]. RB Cavalcante, KM Pereira, CF Nonaka, RL Nogueira, LB de Souza, Immunohistochemical expression of MMPs 1, 7, and 26 in syndrome and nonsyndrome odontogenic keratocystsOral Surg Oral Med Oral Pathol Oral Radiol Endod 2008 106(1):99-105. [Google Scholar]
[4]. M Shear, The aggressive nature of the odontogenic keratocyst: is it a benign cystic neoplasm? Part 1.Clinical and early experimental evidence of aggressive behaviourOral Oncol 2002 38:219-26. [Google Scholar]
[5]. Y Kubota, S Nitta, S Oka, S Nakagawa, T Ninomiya, K Shirasuna, Discrimination of ameloblastomas from odontogenic keratocysts by cytokine levels and gelatinase species of the intracystic fluidsJ Oral Pathol Med 2001 30:421-27. [Google Scholar]
[6]. PA Brennan, B Conroy, AV Spedding, Expression of inducible nitric oxide synthase and p53 in oral epithelial dysplasiaOral Surg Oral Med Oral Pathol Oral Radiol Endod 2000 90:624-29. [Google Scholar]
[7]. Kleinert Hartmut, Pautz Andrea, Regulation of the expression of inducible nitric oxide synthaseEuropean journal of pharmacology 2004 41:255-66. [Google Scholar]
[8]. S Ambs, WG Merriam, WP Benett, Relationship between p53 mutation and inducible nitric oxide synthase expression in human colorectal cancerJ Natl Cancer Inst 1999 91:86-88. [Google Scholar]
[9]. H Kumamoto, T Suzuki, K Ooya, Immunohistochemical analysis of inducible nitric oxide synthase (iNOS) and heat shock proteins (HSPs) in ameloblastomasJ Oral Pathol Med 2002 31:605-11. [Google Scholar]
[10]. Chen Wei-liang, MD DDS, MBA Ouyang Ke-xiong, Expression of Inducible Nitric Oxide Synthase and VascularEndothelial Growth Factor in AmeloblastomaJ Craniofacial Surg 2009 20:171-75. [Google Scholar]
[11]. Agaram Neoplastic status of odontogenic keratocystArch Pathol Lab Med 2004 128:32-8. [Google Scholar]
[12]. SS Noorieh, Zartab Hamed, B Shahab, Immunohistochemical comparison of the expression of p53 and MDM2 Proteins in ameloblastomas and Keratocystic Odontogenic TumoursThe J of Craniofacial Surgery 2011 :22 [Google Scholar]
[13]. CO Rodini, AC Batista, TJ Dionisio, CF Santos, FQ Cunha, VS Lara, Morphologic evaluation and expression of matrix metalloproteinases-2 and 9 and nitric oxide during experimental periodontal disease in ratJ Mol Histo 2008 39:275-82. [Google Scholar]
[14]. LL Thomsen, JM Scott, P Topley, RG Knowles, AJ Keerie, AJ Frend, Selective inhibition of inducible nitric oxide synthase inhibits tumour growth in vivo: studies with 1400W, a novel inhibitorCancer Res 1997 57:3300-104. [Google Scholar]