Prevalence of Carbapenemases and Metallo-β-lactamases in Clinical Isolates of Enterobacter Cloacae
Priyanka Banerjee1, Tavleen Jaggi2, Mehvash Haider3, Bibhabati Mishra4, Archana Thakur5
1Senior Resident, Department of Microbiology, Third Floor, Academic Block, GB Pant Hospital, New Delhi, India.
2Senior Resident, Department of Microbiology, Third Floor, Academic Block, GB Pant Hospital, New Delhi, India.
3Senior Resident, Department of Microbiology,Third Floor, Academic Block, GB Pant Hospital, New Delhi, India.
4Director Professor and Head of Department, Department of Microbiology,Department of Microbiology, GB Pant Hospital, New Delhi, India.
5Director Professor, Department of Microbiology,GB Pant Hospital, New Delhi, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Mehvash Haider, Senior Resident, Department of Microbiology, Third Floor, Academic Block, GB Pant Hospital, New Delhi, India. Phone : 08447560770, E-mail : mehvashaider@gmail.com
Carbapenem resistance, Enterobacter cloacae, Incidence
The emergence of metallo-β-lactamases (MBL’s) in Gram-negative bacilli is increasingly posing a therapeutic threat since the enzymes usually possess a broad hydrolysis profile including carbapenems and extended-spectrum β-lactams [1].
Enterobacter species are among the most common causes of gram-negative health-care associated infections [2]. Resistance to β-lactam antibiotics often complicates the treatment of Enterobacter infections resulting in higher mortality, longer hospitalizations, and higher medical costs [3,4]. The study was undertaken to ascertain the occurrence of carbapenem resistance in E. cloacae. Carbapenem resistance in E. cloacae is unique and various factors such as porin alterations combined with hyperproduction of chromosomal cephalosporinase [5], and production of class A carbapenem- hydrolyzing non metallo-β-lactamases [6] have been described. ESBLs are more difficult to detect in Enterobacter spp. because of AmpC chromosomal enzymes, which are induced by clavulanate, and can then hydrolyse the indicator cephalosporin, thereby masking any synergy arising from inhibition of ESBLs by clavulanate [7].
A total of 68 Enterobacter strains isolated from various clinical samples (blood, ET, drain fluids, pus, others) during a study period of seven months (Feb 2011 to Aug 2011) were included in the study. Species identification was done by the Vitek 2 system. Additional antimicrobial susceptibility testing was done by the Kirby-Bauer disk-diffusion method as per guidelines.
The phenotypic methods used for enzyme detection were Disk Combination Test for ESBL’s (Ceftazidime 30μg and Ceftazidime + Clavulanic Acid 30μg + 10μg), Modified Hodge Test for Carbapenemase [8] and Combined Disk Test with EDTA for Metallo-β-lactamases [8].
Sixty eight Enterobacter species were isolated. Out of 68 strains of Enterobacter spp., 40(58.8%) strains of E.cloacae were isolated followed by 25 (36.7%) strains of E.aerogenes. Three strains of E.dissolvens were also isolated [Table/Fig-1].
Bar diagram showing number of carbapenem resistant strains of E.cloacae and E.aerogenes
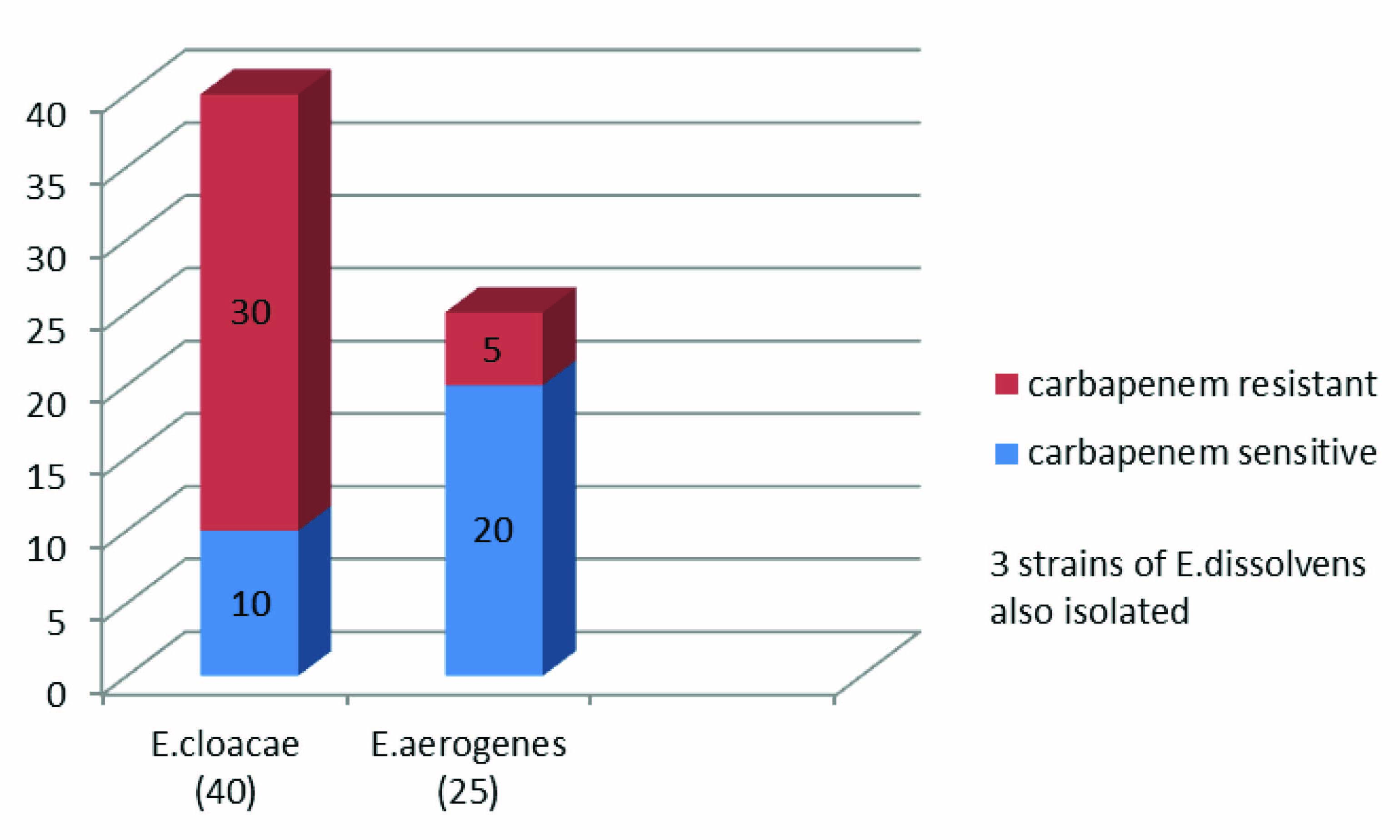
Carbapenem resistance was detected in 30 (75%) isolates of E.cloacae and 5 (20%) isolates of E.aerogenes. Majority (40%) of the resistant isolates were from endotracheal secretion. Majority of the patients had undergone surgery under specialties of Gastroenterology or Neurology.
The mean time from admission to isolation of carbapenem resistant E. cloacae was 14±5 d. Of the 30 strains of carbapenem resistant E.cloacae, 24 (80%) were seen to produce carbapenemase. Production of metallo-β-lactamases was detected in 20 (83.3%) of the 24 carbapenemase producing strains. The rest 4 carbapenemase producing strains were seen to be negative for metallo-β-lactamases suggesting the presence of KPC like carbapenemase. Also, presence of ESBL’s was seen in only 11 (36.6%) strains. Mortality was higher in patients with Carbapenem resistance (33% vs 26%).
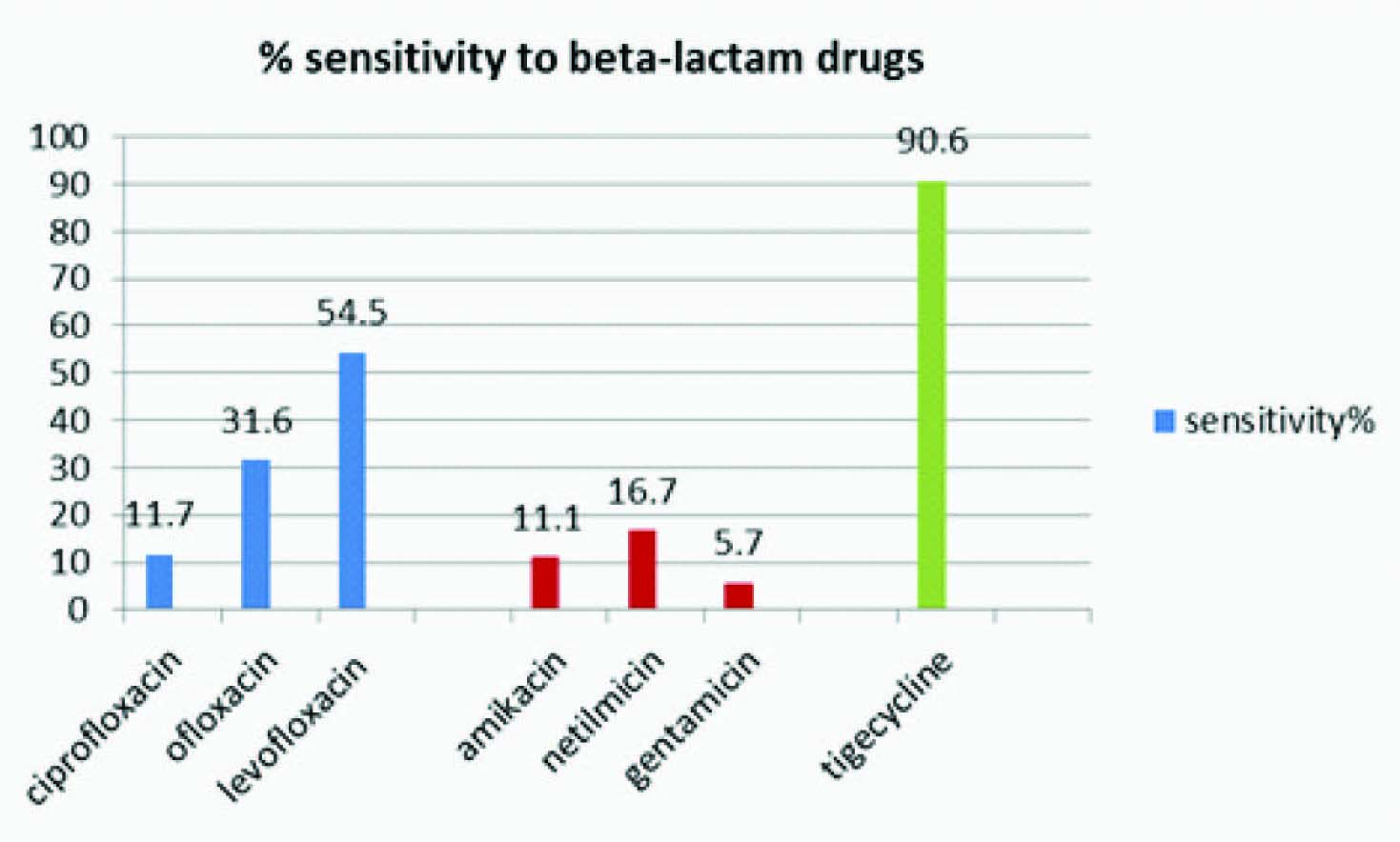
MIC of Carbapenem resistant strains ranged from 4-16μg/ml and that in sensitive strains from 0.25-2μg/ml. Maximum sensitivity was seen towards Tigecycline (90.6%), followed by Levofloxacin (54.5%) [Table/Fig-2]. The strains were uniformly resistant to beta-lactams.
Bar diagram showing percentage of Enterobacter strains sensitive to various antibiotics
Repeated nosocomial outbreaks of infections can be attributed to the ability of E.cloacae to spread among patients [9]. In E.cloacae, reduced outer membrane permeability is associated with reduced susceptibilities to β-lactams and some non-β-lactam antibiotics and [5,10] in conjunction with derepression of intrinsic AmpC cephalosporinases [5].
In this study, we have studied the prevalence of carbapenem resistant Enterobacter in our hospital and also the emergence of metallo-β-lactamase producing strains of E.cloacae. The resistant strains showed 0% sensitivity to β-lactam drugs and maximum sensitivity to Tigecycline(90%). This correlates with the in vitro activity of tigecycline against MBL-producing organisms as documented by some other authors [11]. 33% of the patients with carbapenem resistant strains met with a fatal outcome as compared to 26% in patients with carbapenem sensitive strains. Similar effects on patient outcomes were observed in imipenem-resistant isolates of Enterobacteriaceae in some other studies (Marchaim et al.,) [2,12]. The carbapenems are the last resort for the treatment of resistant Enterobacteriaceae. Thus, recent reports on carbapenem -resistant Enterobacteriaceae are a matter of great concern.
Emergence of carbapenem resistant Enterobacter cloacae is on the rise with the production of carbapenemase being the predominant cause of carbapenem resistance. With limited therapeutic options, the antibiogram should be the guide for the implementation of antibiotic therapy. Prevention of MDR related infections will aid in the reduction of overall morbidity. Timely detection and compliance to infection control measures is the key to prevention of spread.
According to the current recommendations, Cefepime is considered to be the preferred substrate for the detection of ESBL in Enterobacter spp. Due to supply constraints we were unable to comply.
DNA finger printing for epidemiological study and further molecular studies need to be done for confirmation.
[1]. Jing-Jou Yan, Wen-Chien Ko, Chin-Luan Chuang, Jiunn-Jong Wu, Metallo-β-lactamase-producing Enterobacteriaceae isolates in a university hospital in Taiwan: prevalence of IMP-8 in Enterobacter cloacae and first identification of VIM-2 in Citrobacter freundiiJournal of Antimicrobial Chemotherapy 2002 50:503-11. [Google Scholar]
[2]. Marchaim Dror, Navon-Venezia Shiri, Schwaber Mitchell J, Carmeli Yehuda, Isolation of Imipenem-Resistant Enterobacter Species: Emergence of KPC-2 Carbapenemase, Molecular Characterization, Epidemiology, and OutcomesAntimicrobial Agents and Chemotherapy 2008 52(4):1413-18. [Google Scholar]
[3]. Kaye Keith S, Cosgrove Sara, Harris Anthony, Eliopoulos George M, Carmeli Yehuda, Risk Factors for Emergence of Resistance to Broad-Spectrum Cephalosporins among Enterobacter sppAntimicrobial Agents Chemotherapy 2001 45(9):2628-30. [Google Scholar]
[4]. Kang Cheol-In, Kim Sung-Han, Park Wan Beom, Lee Ki-Deok, Kim Hong-Bin, Oh Myoung-don, Bloodstream Infections Caused by Enterobacter Species: Predictors of 30-Day Mortality Rate and Impact of Broad-Spectrum Cephalosporin Resistance on OutcomeClinical Infectious Diseases 2004 39:812 [Google Scholar]
[5]. Lee EH, Nicolas MH, Kitzis MD, Pialoux G, Collatz E, Gutmann L, Association of two resistance mechanisms in a clinical isolate of Enterobacter cloacae with high-level resistance to imipenemAntimicrobial Agents and Chemotherapy 1991 35(6):1093-98. [Google Scholar]
[6]. Rasmussen BA, Bush K, Keeney D, Yang Y, Hare R, O’Gara C, Characterization of IMI-1 beta-lactamase, a class A carbapenem-hydrolyzing enzyme from Enterobacter cloacaeAntimicrob Agents Chemother 1996 40:2080-86. [Google Scholar]
[7]. Crowley B, Ratcliffe G, Extended-spectrum beta-lactamases in Enterobacter cloacae: underestimated but clinically significant!J Antimicrob Chemother 2003 51:1316-17. [Google Scholar]
[8]. Lee K, Chong Y, Shin HB, Kim YA, Yong D, Yum JH, Modified Hodge and EDTA-disk synergy tests to screen metallo-β-lactamase-producing strains of Pseudomonas and Acinetobacter speciesClin Microbiol Infect 2001 7:88-91. [Google Scholar]
[9]. Anderson B, Nicholas S, Sprague B, Campos J, Short B, Singh N, Molecular and descriptive epidemiology of multidrug resistant Enterobacteriaceae in hospitalized infantsInfect Control Hosp Epidemiol 2008 29:250-55. [Google Scholar]
[10]. Werner V, Sanders CC, Sanders WE, Goering RV, Roles of β-lactamases and outer membrane proteins in multiple β-lactam resistance of Enterobacter cloacaeAntimicrobial Agents and Chemotherapy 1985 27:455-59. [Google Scholar]
[11]. Souli M, Kontopidou FV, Koratzanis E, Antoniadou A, Giannitsioti E, Evangelopoulou P, In vitro activity of tigecycline against multiple-drug-resistant, including pan-resistant, gram-negative and gram-positive clinical isolates from Greek hospitalsAntimicrob Agents Chemother 2006 50:3166-69. [Google Scholar]
[12]. Schwaber M, Klarfeld-Lidji S, Navon-Venezia S, Schwartz D, Leavitt A, Carmeli A, Presented at the 47th Interscience Conference on Antimicrobial Agents and Chemotherapy 2007 Chicago, IL [Google Scholar]