Background: Recurrent pregnancy loss (RPL) is one of the most frustrating and difficult areas in reproductive medicine, because the aetiology is often unknown and there are few evidence-based diagnostic and treatment strategies. RPL diagnosis is mainly focused on the female partner. The male factor contributing in evaluation of RPL has been less investigated, it is restricted to karyotype and basic semen analysis, assessment of functionality of sperm is largely ignored.
Aim and Objective: To investigate the role of sperm factors in RPL through regular semen analysis preceded with sperm function tests.
Materials and Methods: We performed a case control study of 95 males whose partner has experienced two or more pregnancy loss as case and 37 volunteers who had fathered child/children without the history of RPL as control group. Basic semen analysis and sperm function test (Nuclear chromatin decondensation {NCD}, Hypo osmotic swelling {HOS} and Acrosome intactness test {AIT} was performed. The results were analysed by performing Independent-sample t-test using SPSS (version 14.0).
Results: One individual had anatomical abnormality which was confirmed through trans-rectal ultrasound scanning and RPL group showed statistically significant (p<0.05) value for NCD, HOS and AIT and 36.8% of RPL individuals had reduced score for sperm count and motility. Less than 4% normal morphology was recorded in 16.8% individuals of RPL group.
Conclusion: Our study revealed that the positive association of sperm dysfunction in RPL cases, hence male may be considered for a routine part of the evaluation along with his partner in the near future in order to achieve desirable outcome.
Introduction
Reproduction in human being is genetically risky process and terribly incompetent. The most common outcome of conception is embryonic or fetal death. Nearly one-third of conceptions do not result in the delivery of a baby. Repeated pregnancy loss in reproductive age couples is a common growing clinical problem that needs adequate attention and investigation. Approximately 15-20% of clinically recognizable pregnancies end in spontaneous abortion during first trimester of pregnancy and there are many more pregnancies that fail prior to being clinically recognized [1]. Recurrent pregnancy loss (RPL), also referred as recurrent miscarriage or habitual abortion and is defined three or more consecutive abortions before the 20th week of gestation [2]. American Society of Reproductive Medicine [3] has recently redefined RPL as two or more abortions. Only 30% of all conceptions result in a live birth [4]. The risk of miscarriage is 30% after two previous losses and 35% after third one. This suggests a need for evaluation after two losses. Studies have revealed that 1 to 2% of women experience RPL [5].
RPL can be considered as primary or secondary: primary RPL refers to couples without previous live birth, while in secondary RPL, a live birth has occurred at some time [6,7]. Various aetiologies which are contributing to RPL like anatomical, psychological, endocrine, infectious, thrombotic, genetic or immunological factors have been extensively studied in females. Even after an extensive diagnosis more than 50% of cases remains obscure [8]. This is a challenging dilemma for both patient and physician.
Chromosomal abnormalities in egg or sperm are associated with embryonic developmental arrest or implantation failure resulting in early pregnancy loss. According to recent studies paternal age plays a vital role in miscarriage where males above 35 y are at increased Malini2risk of RPL [9]. About 30-40% males in reproductive age group have qualitative or quantitative defect in sperm production [10]. There is evidence that male factors play a vital role on fertilization, viability, human embryo development and placental proliferation [11,12]. Centrosome in the first mitotic division after fertilization is contributed by paternal genome [13]. Though 50% of the genomic material is contributed by male gamete to the embryo [14-15] it has been poorly evaluated in RPL cases. Relation between semen parameters and recurrent miscarriage remains controversial till date [16-17]. Limited studies are available in literature with respect to the role of sperm factors and RPL. In this regard we made an attempt to evaluate the male factors like semen quality through the evaluation of routine semen parameters and sperm function tests in RPL males and was compared with control group to evaluate whether there is an increased frequency of abnormal sperm factors in RPL group.
Materials and Methods
Study population
The study was conducted after obtaining Institutional ethical committee clearance (IHEC-UOM No.52/Ph.D/2011-12). This case-control study was conducted for two years at Molecular Reproductive and Human Genetics laboratory, University of Mysore, Mysore. All the subjects were recruited after informed written consent was obtained. Subjects were ascertained through gynecologist of different hospitals in and around Mysore. A detailed family, occupational, reproductive and clinical histories were recorded through pre designed performa. For the present study 95 males subjects aged 20-45 y where their female partners experienced two or more consecutive pregnancy losses without previous live birth (primary RPL) were recruited. The pregnancy losses ranged from 2-7. Thirty seven volunteers who had fathered child/children without the history of recurrent pregnancy loss and unassisted pregnancies are considered as control group.
Semen analysis
Semen samples were obtained by masturbation from both subject and control after recommending three days of sexual abstinence according to the WHO guidelines [18]. Samples were collected in sterile plastic container and allowed to liquefy at 37°C for 30 min and was analysed within one hour of retrieval. The analysis includes physical examination like colour, odour, pH, liquefaction time and volume. Initial microscopic investigations were made to estimate sperm concentration, agglutination of spermatozoan and presence of cellular elements other than spermatozoa. Routine semen analysis like sperm count were performed using Neubauer counting chamber (expressed in millions of spermatozoa Per millimetre). Percentage of rapidly progressive motile spermatozoa (a) and sluggish progressive motile spermatozoa (b). Viability was recorded using Eosin-nigrosin stains (a modified Blom’s technique) [Table/Fig-1] and morphology was examined by Papanicolaou staining method. All samples were evaluated according to WHO (2010) guidelines.
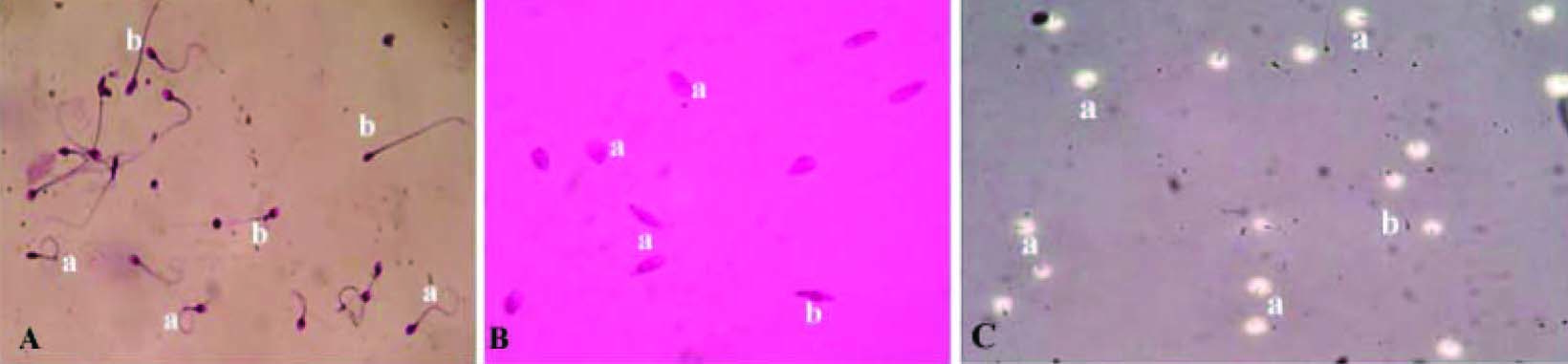
Sperm function test [Table/Fig-2a-c]
Nuclear chromatin decondensation test (NCDT )[19]: This test was carried out to check the ability of nuclear material to decondense in vitro in spermatozoa. Semen sample was centrifuged to separate plasma. The pellets were washed with 0.05M borate buffer. One volume of sample was mixed with nine volumes of EDTA-SDS mixture and incubated at 370C for 60 min. An equal volume of glutaraldehyde borate buffer was added. A drop of this mixture was transferred on to clean glass slide and covered with cover slip and observed under microscope in 400X magnification. The number of condensed and decondensed heads was counted. If more than 70% of spermatozoa show decondensed nuclear chromatin then it was considered as normal [Table/Fig-2].
Hypo-osmotic swelling test (HOST) [20]: Integrity of plasma membrane was performed using this test. Hypo-osmotic solution was prepared using fructose and sodium citrate in equal proportion. One ml of this solution was incubated at 370C for 10 min and 100μl of semen sample was mixed. It was incubated at 370C for 30 min. This mixture was dropped on pre-cleaned glass slide, covered with cover slip and observed under microscope in 400X magnification. Percentage of coiled (curled) tail was recorded. If more than 60% of spermatozoa, shows coiled tail then it was considered as normal [Table/Fig-2].
Acrosomal intactness test (AIT) [21]: Quality of the enzyme in the acrosome was analysed using this test. PBS –d-glucose was prepared. Gelatin coated slides were prepared by spreading warm aqueous solution of gelatin on to a clean glass slide and kept horizontally at 400C for 24 h. These coated slides were immersed in PBS-glutaraldehyde solution for 2 minutes and washed using distilled water and stored at 400C. Semen was mixed with PBS- D-glucose in the ratio of 1:5 and incubated at 370C for 10 min. Gelatin coated slides were allowed to come to room temperature. A drop of diluted semen samples was smeared over gelatin slide. This slide was placed on to a petri dish containing a moistened filter paper and incubated for two hours at 370C. The slides were examined under phase contrast microscope in 400X magnification. The percentage spermatozoa with halos surrounding the head were recorded. Values more than 50% was considered as normal [Table/Fig-2].
Statistical Analysis
Statistical analysis was performed by using statistical program SPSS (version 14.0). Independent- Sample t-test was performed to find out whether any significant mean difference exist between the case and control. Results were reported as mean ± standard deviation. Significant differences were considered when p-value is less than 0.05.
Results
The mean age of males of the RPL and control group was 33.06 ± 5.75 and 30.02±6.84 respectively. Mean number of abortion in the RPL group was 2.72 ± 1.03. One individual had anatomical abnormalities like cyst in the head of the epididymis and small right testis but had a normal count with subnormal motility (<50) and normal value for sperm function test and had a history of two miscarriages. Physical parameters in RPL and control group are depicted in [Table/Fig-3]. Grades of agglutination were observed in 17.8% of the cases. Basic semen parameters in RPL and control group are depicted in [Table/Fig-4]. For the routine semen parameters 36.8% individuals showed abnormality in RPL group. Subnormal sperm morphology (teratozoospermia) i.e. less than 4% normal sperm was recorded in 16.8% individuals. In our study out of 95 cases five cases had oligospermia, four cases with oligoasthenoteratozoospermia, teratozoospermia and oligoasthenospermia, six cases with asthenoteratozoospermia and oligoteratozoospermia, eight cases with asthenozoospermia.
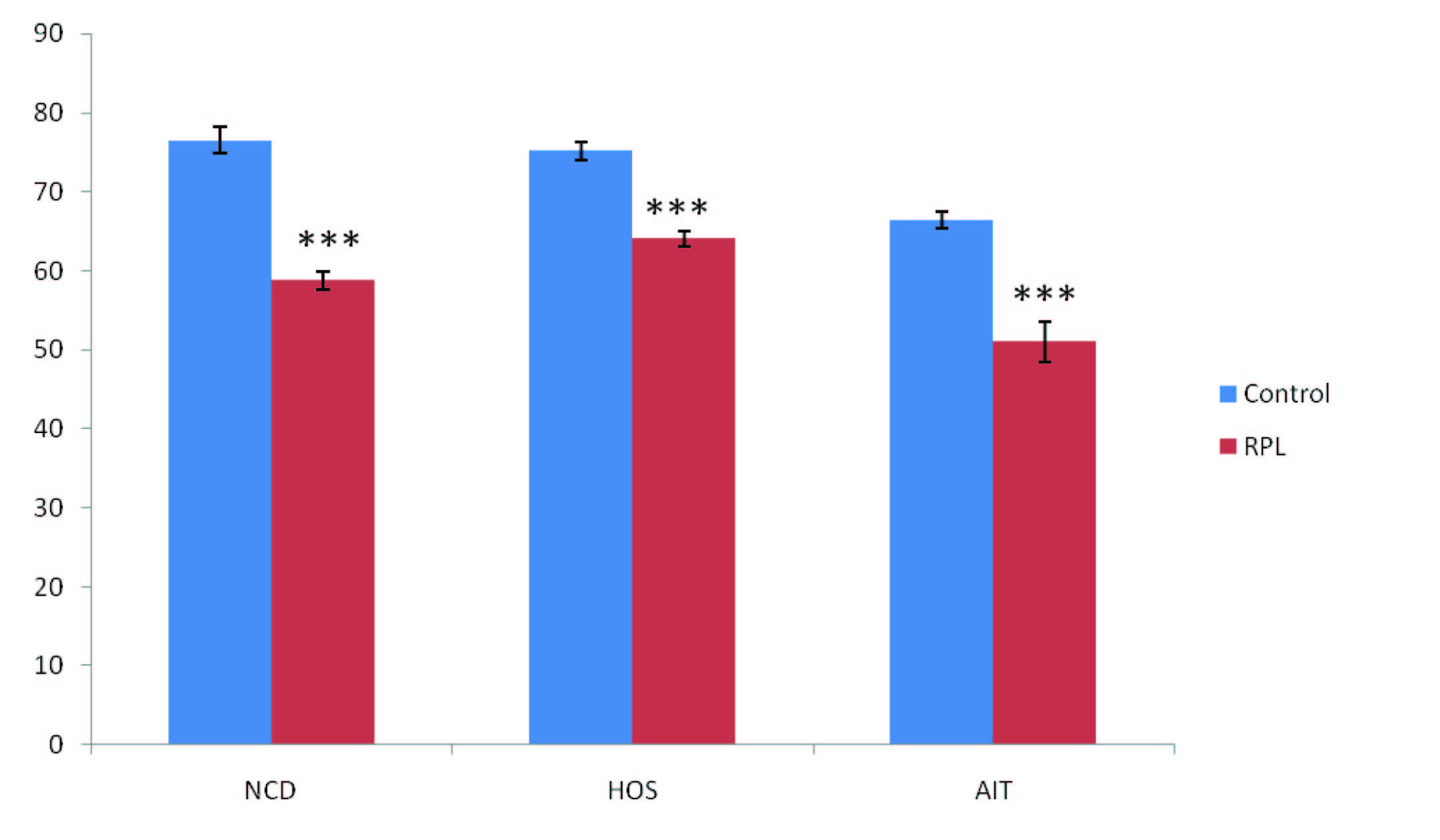
Apart from the routine semen analysis specific investigations are required to access the functional status of sperm and these includes NCD, HOS and AIT. Sperm function test scores were shown in [Table/Fig-5]. For sperm function test 58.9% of the cases showed abnormal value for NCD (<70), 23% of the cases showed abnormal value for HOS (<60%) and abnormal scores for AIT was recorded in 15% of the cases (<50). Out of 16 teratozoospermic cases 10, 4 and 3 individuals showed sub normal scores for NCD, HOS and AIT respectively.
Discussion
The word miscarriage is misleading, because it seems that only females are involved in its nature. But it is well recognized that both couples are involved since male gamete contributes one-half of the genome content to the embryo. The relation between standard semen parameters and recurrent pregnancy loss has been controversial subject [22]. Few researchers did not find any significant difference in the semen parameters like count, motility and morphology between recurrent spontaneous abortion and control group [17,18]. Buckett et al., [23] showed correlation with HOS test in recurrent miscarriage group and also showed the viability and quality of the sperm may have an impact on conception and miscarriage rates. Carrell et al., [24] reported decreased percentage of morphologically normal sperm in the recurrent pregnancy loss patients compared with both the general population, control group and the donors. The differences were observed for percentage of amorphous sperm, hypo-osmotic solution reactivity between the recurrent pregnancy loss group and the donors of known fertility and also increased sperm DNA fragmentation in RPL patients
Gopalkrishnan et al., [25] studied sperm nuclei and sperm chromatin condensation in greater detail and observed increased sperm nuclear vacuoles and abnormal chromatin condensation in RPL group. Few IVF studies showed association of high abnormal sperm morphology with embryo failure at an early cleavage stage [26,27]. Recent study by Absalan et al., [28] reported significant difference in sperm morphology and motility between RPL and control group.
The intactness of the plasma membrane was evaluated by HOS test. It is not only an indicator of the chemical integrity of the plasma membrane but also its physical integrity. Buckett et al., [23] showed correlation with HOS test in recurrent miscarriage group. Twenty two (23%) individual in RPL group had low scores for HOS and the mean scores of both the groups were within the normal range.
NCD of spermatozoa and subsequent male pronucleus formation is essential for fertilization and normal embryonic development. The failure of sperm decondensation in the oocytes may be a consequence of a subtle sperm abnormality like structural or biochemical defects associated with chromatin packaging or organization during spermatogenesis [29]. Chromatin damage precedes the loss of fertilization potential and poor embryo quality, resulting in pregnancy loss. The mean score for NCD test are subnormal in RPL group and 56 (58.9%) individual in RPL group had lesser score. Absalan et al., [28] tested the sperm DNA fragmentation by sperm chromatin depression (SCD) test and they observed significant difference between RPL and control group.
Acrosin and Hyaluronidase are the two main acrosomal enzymes (proteases), which plays an important role in penetration of spermatozoa through outer membrane of oocyte. In our study mean AIT scores were observed to be within normal range in both RPL and control group. Subnormal scores were observed in 14(15%) individuals of RPL group. Saxena et al., [29] reported mean less scores for all the three function tests in RPL group when compared to controls. The pilot study carried out by Chaithra et al., [30] reported significantly lower scores for semen profile and sperm function tests in the RPL group when compared to the control group.
This study strengthens the current literature associating sperm quality with RPL, and emphasizes evaluating male factor by sperm function tests along with conventional semen parameters. Though this study has difference in the ratio of cases and control sample number and may not show a aetiology and the effect co-relation, but the significant association of the abnormal sperm parameters and sperm dysfunction suggest a possible cause. Taken together our data suggest that though individual has normal sperm count, motility and morphology but the sperm function tests exhibit sub normal scores which may be the possible aetiology for RPL. Hence, we recommend screening of both partners simultaneously in RPL case to achieve desirable outcome. It might also assist in the selection of functionally superior sperm of such male partners who opt for assisted reproductive techniques like intra-cytoplasmic sperm injection (ICSI) or in vitro fertilization (IVF). In future such studies are required with huge number of case and control subjects to ascertain the specific sperm dysfunction which is predominate in causing RPL.
Sperm viability test- Unstained sperms indicates viable sperm and stained sperms are inviable sperms
Sperm function tests- Plate A: Hypo-osmotic swelling test: coiled tail ‘a’ indicates positive response uncoiled tail ‘b’ indicates negative response. Plate B: Nuclear chromatin decondensation test swollen head ‘a’ indicates positive response and ‘b’ indicates negative response. Plate C: Acrosome intactness test formation of halo surrounding sperm head ‘a’ indicates positive response and negative response is indicated by ‘b
| Physical Parameters | RPL group n=95 Mean ± SD | Control group n=37 Mean ± SD |
| Ejaculate volume (ml) | 2.29 ± 1.21 | 2.48±1.17 |
| pH | 7.92 ± 0.42 | 7.81 ± 0.26 |
| Semen parameters | Mean± SE | Control group n=37 Mean± SE | p-value |
| Count(x106/ml) | 46.35± 3.23 | 56.13±4.83 | 0.894 |
| Motility (a+b%) | 58.42±2.12 | 62.91±1.76 | 0.001** |
| Viability | 63.89±1.36 | 75.02±0.92 | 0.001** |
(**=Statistical significant)
Sperm function tests in control and RPL group. NCD, HOS and AIT showed significant decreased value in RPL group when compared to control with p- value <0.05(***), Note: X-axis-Sperm function test, Y-axis-In percent
NCD: Nuclear chromatin decondensation, HOS: Hypo osmotic test, AIT: Acrosome intactness test
Acknowledgement
We are indebted to all subjects and gynecologists for their cooperation. The authors thank our research team in Molecular reproductive and Human genetics laboratory, University of Mysore, for their support. We also thank the Chairman of the Department of Studies in Zoology for providing facilities to conduct the experiments. KP is thankful to UGC-RFSMS (BSR) for financial support.
(**=Statistical significant)
[1]. J Dejmek, J Vojtassak, J Dejmek, Cytogenetic analysis to 1508 spontaneous abortions originating from South SlovakiEur J Obstet Gynecol Reprod Biol 1992 46:129-36. [Google Scholar]
[2]. HB Ford, DJ Schust, Recurrent pregnancy loss: aetiology, diagnosis, and therapyRev Obstet Gynecol 2009 2(2):76-83. [Google Scholar]
[3]. Practice Committee of the American Society for Reproductive Medicine. Evaluation and treatment of recurrent pregnancy loss: A committee opinion. Fertil Steril 2012 98:1103 [Google Scholar]
[4]. NS Macklon, JP Geraedts, BC Fauser, Conception to ongoing pregnancy: the ‘black box’ of early pregnancy lossHum Reprod Update 2002 8(4):333-43. [Google Scholar]
[5]. MD Stephenson, Frequency of factors associated with habitual abortion in 197 couplesFertil Steril 1996 66(1):24-29. [Google Scholar]
[6]. AH Ansari, B Kirkpatrick, Recurrent pregnancy loss. An updateJ Reprod Med 1998 43:806 [Google Scholar]
[7]. M Paukku, M Tulppala, M Puolakkainen, Lack of association between serum antibodies to Chlamydia trachomatis and a history of recurrent pregnancy lossFertil Steril 1999 72:427 [Google Scholar]
[8]. American Society for ReproductiveMedicine (ASRM). Recurrent pregnancy loss. Patient’s Fact Sheet. Created February 2005. Available at: http://www.asrm.org/Patients/FactSheets/fact.html [Accessed January 9, 2008] [Google Scholar]
[9]. K Kleinhaus, M Perrin, Y Friedlander, O Paltiel, D Malaspina, S Harlap, Paternal age and spontaneous abortionObstet Gynecol 2006 108:369-77. [Google Scholar]
[10]. C Krausz, K McElreavey, Y chromosome and male infertilityFront Biosci 1999 15:1-8. [Google Scholar]
[11]. M Moomjay, LT Colomber, LL Veeck, Z Rosenwaks, GD Palermo, Sperm integrity is critical for normal mitotic division and early embryonic developmentMol Hum Reprod 1999 5:836-44. [Google Scholar]
[12]. JH Check, D Karsoff, ML Check, Some semen abnormalities may cause infertility by impairing implantation rather than fertilizationMed Hypotheses 2001 56(5):653-57. [Google Scholar]
[13]. AH Sathananthan, Mitosis in the human embryo: the vital role of the sperm centrosome (centriole)Histol Histopathol 1997 12:827-56. [Google Scholar]
[14]. M Miozzo, G Simoni, The role of imprinted genes in fetal growth Biol Neonate 2002 81:217-28. [Google Scholar]
[15]. EE Puscheck, RS Jeyendran, The impact of male factor on recurrent pregnancy lossCurr Opin Obstet Gynecol 2007 19:222-28. [Google Scholar]
[16]. JA Hill, DJ Anderson, K Polgar, AF Abbott, JA Politch, Seminal white blood cells and recurrent abortionHum Reprod 1994 9:1180-83. [Google Scholar]
[17]. Sbracia M, Cozza G, Grasso J.A., Mastrone M, Scarpellini F, TSemen parameters and sperm morphology in men in unexplained recurrent spontaneous abortion, before and during a 3 year follow-up periodHuman Reproduction 1996 11(1):117-20. [Google Scholar]
[18]. World Health Organization. Laboratory manual for the examination of human semen and semen cervical mucus interaction 1999 4th EditionNew York:Cambridge University Press, Cambridge, UK [Google Scholar]
[19]. K Gopalkrishnan, IN Hinduja, TC Anandkumar, In vitro decondensation of nuclear chromatin of human spermatozoa assessing fertilizing potentialArch Androl 1991 28:43-50. [Google Scholar]
[20]. Jeyendran R. S., Ven H. H. Van der, Zaneveld L. J. D., The Hypo-osmotic Swelling Test: An UpdateSystems Biology in Reproductive Medicine 1992 27(2):105-16. [Google Scholar]
[21]. K Gopalkrishnan, D Padwal, V Balaiah, Efficiency of routine semen analysis to predict functional and structural integrity of human spermatozoaIndian Journal of Experimental Biology 1995 33(9):652-54. [Google Scholar]
[22]. A Zini, M Sigman, Are tests of DNA damage clinically useful?Pros and Cons J of Androl 2009 30:220-29. [Google Scholar]
[23]. WM Buckett, MJ Luckas, IA Aird, The hypo-osmotic swelling test in recurrent miscarriageFertil Steril 1997 68:506-29. [Google Scholar]
[24]. DT Carrell, AL Wilcox, Lowy Leasa, CM Peterson, KP Jones, Erickson Lisa, Elevated Sperm Chromosome Aneuploidy and Apoptosis in Patients with Unexplained Recurrent Pregnancy LossThe Am Coll of Obst and Gynec 2003 101(6):1229-35. [Google Scholar]
[25]. K Gopalkrishnan, V Padwal, PK Meherji, Poor quality of sperm as it affects repeated early pregnancy lossArch Androl 2000 45(1):111-17. [Google Scholar]
[26]. TF Kruger, AA Acosta, KF Simmons, RJ Swanson, JF Matta, Predictive value of abnormal sperm morphology in in vitro fertilizationFertil Steril 1988 49:112-17. [Google Scholar]
[27]. JH Check, L Stumpo, D Lurie, K Benfer, C Callan, A comparative prospective study using matched samples to determine the influences of subnormal hypoosmotic swelling test score of spermatozoa on subsequent fertilization and pregnancy rates following in vitro fertilization Hum Reprod 1995 10:1197-200. [Google Scholar]
[28]. F Absalan, A Ghannadi, M Kazerroni, R Parifar, F Jamalzadeh, S Amiri, Value of sperm chromatin dispersion test in couples with unexplained recurrent abortionGamete biology 2012 29:11-14. [Google Scholar]
[29]. P Saxena, MM Misro, SP Chaki, K Chopra, S Roy, D Nandan, TIs abnormal sperm function an indicator among couples with pregnancy loss?Fertil Steril 2008 90(5):1854-58. [Google Scholar]
[30]. PT Chaithra, G Sreenivasa, VS Vineeth, P Kavitha, Najafi Mohsen, kumar Sharat, Male Factors: An Ignored Aetiology in Recurrent Pregnancy LossWorld J Life Sci. and Medical Research 2011 1(4):76-82. [Google Scholar]