The success of endodontic treatment therapy depends on how well we eliminate pathogenic microflora from the root canal system and this remains our ultimate goal. Although various fungi, viruses, archaea contribute to the microbial diversity in endodontic infections, but the most common micro organism occurring in these infections are bacteria’s [1]. Some microorganisms are resistant to antimicrobial treatment, it includes various Gram negative anaerobic rods such as Fusobacterium nucleatum, Prevotella species and gram positive bacterias such as Streptococcus gordonii, Enterococcus faecalis, Actinomyces species, etc [2]. The Microbial infection produce by these organisms plays an important role in the development of necrosis in dental pulp and leading to the formation of periapical lesions [3].
During chemomechanical preparation of the canal, various endodontic irrigants, such as sodium hypochlorite, iodine potassium iodide, chlorhexidene, etc are available, each having its own certain advantages with some limitations [4] and however specially in inner layers of dentin they do not achieve a complete disinfection [5].
Scanning electron microscopic investigations have demonstrated and revealed the penetration of bacteria up to 1000 μm into dentinal tubules in a laboratory model and hence it is very difficult for normal irrigants to penetrate till this depth. Sodium hypochlorite can penetrate in a range of 60- 150 micro meters into dentinal tubules and of Nd:YAG Laser is in a range of 400- 850 micro meter [6].
As the success of endodontic treatment depends on proper debridement and disinfection of root canal, today several newer modalities or devices are available and they must be tried, tested and used for disinfection of the root canal. Recently Novel approaches for disinfection of root canals have been proposed that include the use of high power lasers and photodynamic therapy [7].
Photodynamic therapy (PDT) uses photoactive dye (photosensitizer) which in the presence of oxygen gets activated on exposure to light of a specific wave length. The energy which is transferred from the activated photosensitizer to available oxygen results in to the formation singlet oxygen and free radicals which are toxic oxygen species. These highly reactive chemical species can damage proteins, lipids, nucleic acids, and other cellular components. The most important property of PDT is that the bacteria present in root canal system do not develop resistance to PDT. It is effective on viruses, fungus and protozoa, kills bacteria rapidly and works instantaneously. Heat production during the exposure is less, no side effects. Because of this the applications of PDT in dentistry are growing rapidly for several treatment modalities as treatment of oral cancer, the treatment of oral lichen planus, leukoplakia and head and neck cancer, bacterial and fungal infection therapies. Photodynamic antimicrobial chemotherapy has also being effective in the treatment of bacterial, fungal, parasitic and viral infections [8].
So, in order to get a proper disinfection of root canals and for success of endodontic treatment photodynamic therapy can be of great help as photosensitiser, as it possess a pronounced cationic charge that rapidly bind and penetrate bacterial cells and therefore these compounds show a high degree of selectivity for killing microorganisms compared to host mammalian cells [7].
In the present study we evaluated the effectiveness of PDT using Methylene blue dye and red light emitted by diode laser in the wavelength of 910 nm against Enterococcus faecalis, by counting the colony forming units
Materials and Methods
The present study was conducted in Teerthanker Mahaveer Dental College & Research Centre. The study was conducted in the month of June 2014 within the period of 24 days. Initially 21 d inoculation of sample was done. Then on next day disinfection procedure of sample was done through photodyanamic therapy. Followed by disinfection next two days, scanning electron microscope study was carried to obtain the desired results.
Firstly teeth required for study was collected from Department of Oral and Maxillofacial Surgery. Only freshly extracted 20 intact, non carious single rooted teeth which were indicated for orthodontic treatment were taken for this study. The single rooted teeth were considered for ease of processing specimens and availability. Statistical analysis was done using Student’s Unpaired t-test were at (p<0.001) was found to be highly significant.
Inclusion Criteria
- Non Carious teeth
- Single Rooted teeth
Exclusion Criteria
- Carious, Deciduous Teeth, Dilacerated Roots
- Multirooted Teeth
Preparation of specimen
Firstly the collected teeth were cleaned ultrasonically, stored in sodium azide solution till they were processed. Then they were autoclaved keeping the parameters as 121oC for 15 min at the pressure of 15psi. Followed by this the access openings were done immediately after autoclaving and canals were negotiated with 15# K – file.
Then after this cleaning and shaping of the root canals were done till F4 with 3% sodium hypochlorite and 17% Ethylenediaminetetraacetic acid(EDTA) gel. Lastly teeth were decoronated using a diamond disc at cementoenamel junction.
Cultivation and inoculation of bacteria
In the present study, Enterococcus faecalis, ATCC- 29212 bacteria were chosen. The bacteria were grown on petri dish for 24 h on a brain heart infusion agar [Table/Fig-1].
Bacteria’s collection from Agar Plate
Then the Enterococcus faecalis bacteria were collected from the agar plate and added to 1ml of Brain Heart Infusion liquid [Table/Fig-2].
Bacteria’s Transferring into Brain Heart Infusion liquid

Then followed by this Brain Heart Infusion liquid (BHI) media was prepared and then 6 ml of media was taken. 400 micro liters of Enterococcus faecalis suspension was added in 6 ml BHI liquid.
Specimens were suspended in 6 ml BHI liquid media in a glass bottle. The specimens were then incubated at 370C for 21 d in an incubator. The media was changed twice a week [Table/Fig-3,4].
Bacterial colonies after 48 h in Control Group.,

Bacterial colonies after 48 h in PDT Group
Preparation of specimens for experimental groups
- The specimens were kept for 21 d. Then after 21 d, specimens were removed from Brain Heart Infusion liquid media and were thoroughly washed with normal saline.
- Then these specimens were divided into 2 groups; Control Group – 10 samples and PDT group – 10 samples [Table/Fig-5] Now the specimens which were in the control group were left untreated and the specimens who were in PDT group were subjected to Photodynamic therapy, using methylene blue dye and a diode laser.
- Then Methylene Blue (MB) was mixed with phosphate buffered saline to get the final concentration of 25 mg/ml. Entire specimens were fully immersed in MB for 10 min. After 10 min, specimens were removed and washed with normal saline. Once the specimen were washed and dried, they were irradiated with diode laser (910 nm wavelengths with power settings of 1W, at a pulsed mode).
- Optical fiber was then inserted in the root canal of a specimen. The fiber were kept 2 mm short of working length and were kept in contact with root canal walls and it was moved in apico-coronal direction giving circular movements for 20 sec, which was repeated three times with the gap of 10 sec between each one.
- Immediately then samples were transferred to normal saline.

Microbiological Examination
Thereafter, sterile paper points were then placed in the root canal of specimens. After 60 sec paper points were transferred into Brain Heart Infusion liquid for one hour to obtain desired samples for the microbiological examination.
These samples were then placed on BHI Agar plate and incubated for 2 d (48 h). The bacterial count technique was used to assess the total number of viable bacteria in colony forming units per milliliter (CFUs/mL).
Two longitudinal grooves were prepared on buccal and lingual surfaces of specimens with a diamond disc. Using a chisel and mallet the tooth specimens were fractured longitudinally into two halves.
Microscopic examination of specimens
Examination of specimens was done under scanning electron microscope to check the penetration depth of bacteria into tubules.
Results
In this study, the mean values of CFU’s for Control group and the mean value for PDT group was observed after 48 h of incubation.
Percentage of CFU reduction was calculated using the below mentioned formula:
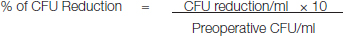
It was found that the mean value of CFU’s for Control group was 60.5 × 106 CFU/ml and mean value for PDT group was 2.0 × 106 CFU/ml. By applying Student’s Unpaired t-test there is a highly significant difference between mean values of colony forming units in Group I and group II (p<0.001) [Table/Fig-6&7].
Number of colonies after 48 h of incubation (CFU/ml) Microbiological examination revealed following values of colony forming units in Control and PDT group
| Control Group | PDT Group |
|---|
| 57 × 106 | 2.4 × 106 |
| 64 × 106 | 2.2 × 106 |
| 55 × 106 | 1.6 × 106 |
| 66 × 106 | 2.8 × 106 |
| 60 × 106 | 1.7 × 106 |
| 61 × 106 | 1.2 × 106 |
| 57 × 106 | 2.3 × 106 |
| 64 × 106 | 1.8 × 106 |
| 52 × 106 | 2.2 × 106 |
| 69 × 106 | 1.8 × 106 |
Comparison of mean and SD values of Colony forming units in Group I and group II
| Colony forming units |
|---|
| Group I (Control) (n=10) Mean ± SD | Group II Photo activated disinfection (n=10) Mean ± SD | Student’s Unpaired ‘t’ test value | p-value and significance |
| 60.5±5.04 | 2.0±0.44 | 40.07 | p<0.001, highly significant |
*By applying Student’s Unpaired t-test there is a highly significant difference between mean values of colony forming units in Group I and group II (p<0.001).
It was found that percentage of CFU reduction in PDT group was 96.70%. Depth of penetration of bacteria’s in control group was around 980μm. In PDT group there were no bacteria’s till 890-900 μm. This was calculated using scanning electron microscope.
The result of the study suggested the potential of PDT to be used as an adjunctive antimicrobial procedure after standard endodontic chemo mechanical debridement.
Discussion
Root canal system having infections typically have a poly microbial flora having both gram positive and gram negative bacterias [9]. It has been demonstrated by Scanning electron microscope that bacterial penetration can be up to 1000 μm into dentinal tubules in a laboratory model.
Due to the complex nature of root canal system it becomes difficult for the complete debridement of canal containing bacteria’s with instrumentation and irrigation alone. Also due to the formation of a smear layer after instrumentation disinfecting the dentinal tubules have found to be less effective by use of irrigants and intracanal medicaments [10]. Because of this reason most of the root canal treatment fails.
There are some gram positive bacteria’s such as Enterococcus faecalis, Lactobacilli, Streptococcus gordonii, Streptococcus mitis, Actinomyces species, etc and some gram negative anaerobic rods such as Prevotella, Fusobacterium nucleatum species which are resistant to antimicrobial treatment and can survive in the root canal after cleaning and shaping of the canal [11]. Hence, newer modalities were tried and tested for thorough disinfection of root canals.
In our study, we studied the effect of Photodynamic therapy on Enterococcus faecalis by incubating the bacteria’s in tooth specimens and then Colony Forming Units (CFU) were calculated and then depth of penetration of Photodynamic Therapy was checked using Scanning electron microscope.
We found that, the control group showed the mean value of 60.5 × 106 CFU/ml. The PDT group showed the mean value of 2.0 × 106 CFU/ml. There was a 97% reduction in bacterial viability by using PDT. Depth of penetration of bacteria’s in the control group was around 980μm. In PDT group there were no bacteria’s till 890-900 μm. The result of the study suggested the potential of PDT to be used as an adjunctive antimicrobial procedure after standard endodontic chemo-mechanical debridement.
Enterococcus faecalis is a gram positive facultative anaerobic organism, commonly isolated from root canal system. Enterococci are hardy inhabitants of the root canal system, which are difficult to eliminate than other taxa by using standard disinfection procedures [12].
Enterococcus faecalis becomes more resistant in starvation phase. It forms biofilms which are difficult to eliminate. Enterococcus faecalis can with stand high pH. Conventional irrigants cannot completely eliminate Enterococcus faecalis, because of lesser penetration depth into dentinal tubules. Enterococcus faecalis is known to colonize dentinal tubules upto depth of 600- 1000 μm [13], whereas conventional irrigants penetrate no more than 100 μm [9].
Brain Heart Infusion media was used to cultivate the bacteria as it is a highly nutritious general purpose growth media. It is made by the recuperation of nutrients from boiled cattle hearts and brains.
Photo Dynamic Therapy was actually developed as a therapy for cancer and is based on the concept that a nontoxic photosensitizing agent, known as a photosensitizer, which can be preferentially localized in premalignant and malignant tissues and subsequently activated by light of the appropriate wavelength to generate singlet oxygen and free radicals that are cytotoxic to cells of the target tissue [14]. Several studies have shown that oral bacterias are susceptible to PDT.
In this therapy, certains dyes such as Toluidine blue, Methylene blue, Azuline paste, Tolonium chloride and light source such as Diode laser, 35-mW helium–neon low power laser [15], Gallium-aluminium arsenide (Ga-Al-As) diode laser can be used.
Methylene blue, an organic dye belonging to the phenothiazine family, has well-established photosensitizing properties and has been used in photodynamic therapy. Very few gram-positive and gram-negative oral bacteria are known to be photo inactivated by methylene blue [10].
The hydrophilicity of methylene blue, along with its low molecular weight and positive charge, allows the passage across the porin-protein channels in the outer membrane of gram-negative bacteria. MB predominantly interacts with the anionic macromolecule lipopolysaccharide, resulting in the generation of MB dimmers, which participate in the photosensitization process [14].
Diode is a soft tissue laser. It is mostly used in periodontal therapies. It is available in various wavelengths such as 810 nm, 940 nm, 980 nm. These wavelengths are absorbed by pigments such as melanin, haemoglobin, chromophores, methylene blue, etc. These wavelengths selectively kill bacterias through cell wall lysis and produce the bactericidal effect.
Methylene blue alone exhibited 83.2% reduction of E. faecalis biofilm species in the root canal system of extracted human teeth. The combined effect of methylene blue and red light causes the generation and diffusion of the reactive free radicals, which causes damage to proteins, a bacterial membrane (made up of lipids) and nucleic acids thus causes bacterial lysis. These reactive free radicals are responsible for the photodynamic effect, which will fully penetrate dentinal tubules, including the conventionally unreachable areas, and eliminate residual microorganisms [10].
Scanning electron microscopic investigations have demonstrated bacterial penetration up to 1000 μm into dentinal tubules in a laboratory model. E. faecalis can penetrate the dentinal tubules to the depth of 1200 micro meters. It is very difficult for normal irrigating agents to penetrate till this depth. The depth of penetration of Sodium hypochlorite into dentinal tubules is in a range of 60-150 micro meters and of Nd:YAG Laser is in a range of 400- 850 micro meter [6].
In our study the bacterias were found till the depth of around 980μm in the control group [Table/Fig-8,9], whereas in PDT group till the depth of 890-900 μm, there were no microorganisms present [Table/Fig-10,11].
Scanning Electron Microscope image of control specimen: Bacteria’s were found upto depth of 980 μm

Scanning Electron Microscope image of bacteria’s in control group
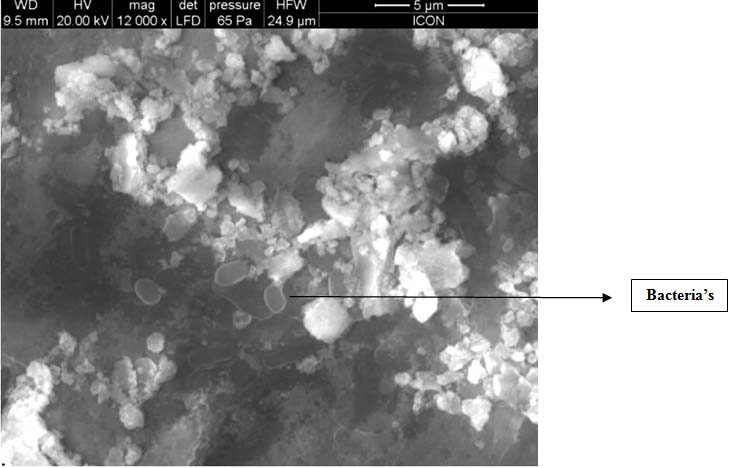
Scanning Electron Microscope image of bacteria in PDT group till the depth of 900μm there were no bacteria’s
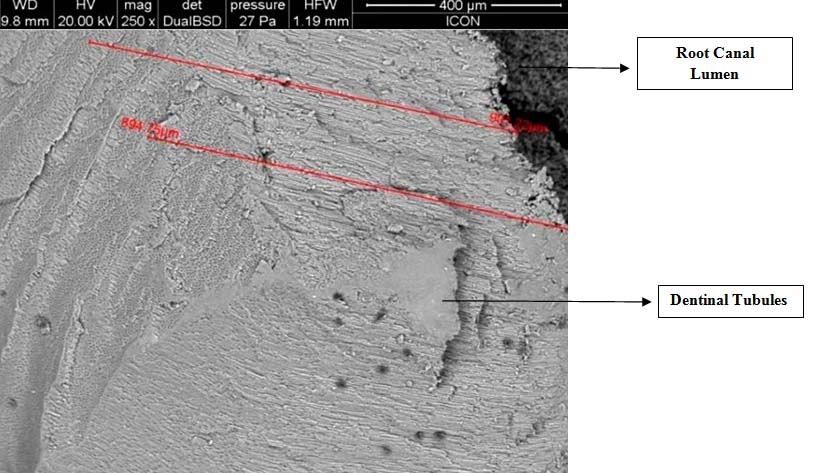
Scanning Electron Microscope image of PDT specimen

Nikolaos et al., [10] concluded that PDT may be developed as an adjunctive procedure to kill residual bacteria in the root canal system after standard endodontic treatment.
Jacob Lee Fimple et al., [14] investigated the photodynamic effects of methylene blue on multispecies root canal biofilms comprising Actinomyces israelii, Fusobacterium nucleatum, Porphyromonus gingivalis and Prevotella intermedia in experimentally infected root canals of extracted human teeth in vitro. They achieved up to 80% reduction of colony-forming units and concluded that PDT can be an effective adjunct to standard antimicrobial treatment.
Vaziri S et al., conducted a study on 60 single rooted teeth and found that combination of Photodynamic therapy and 2.5% NaOCl achieved maximum reduction in recovered viable bacteria. After treatment by PDT + 2.5% NaOCl, no viable bacteria were observed [16].
Javed F et al., stated that PDT significantly helps in reducing periodontopathogenic bacteria including Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis, Prevotella intermedia, etc. PDT kills cariogenic bacteria (such as Streptococcus mutans and Streptococcus sanguis), the bacteria associated with infected root canals, and those associated with peri-implantitis [17].
Garcez AS et al., used PDT along with conventional endodontic treatment in vivo and concluded that PDT leads to major reduction of microbial count. PDT is an efficient treatment to kill micro organisms having multi-drug resistant [18].
One of the studies which showed contrast findings result was conducted in Vivo by Muhammad OH et al., which concluded that there was no significant difference between results obtained from groups which were treated one by Aseptim Plus® and other by Diode Laser (p < 0.6267). The cultures showed there was maximal bacterial growth. It was found that the group that was treated by ultrasonic irrigation and NaOCl and EDTA solutions had the best results (p < 0.0001): there was a statistically significant reduction of bacterial load and destruction of microbial biofilm. Hence in this study, it concluded that Photodynamic therapy could not disrupt endodontic artificial microbial biofilm and also could not inhibit bacterial growth in a clinically favourable working time [19].
Therefore, Photo-activated disinfection is not an alternative but a possible supplement to the existing protocols for root canal disinfection as the interaction between light (diode laser) and associated dye provides a broad-spectrum effect.
Conclusion
The results of present study help us to conclude that the Photodynamic therapy reduces the number of bacteria’s in infected root canals effectively and increases the changes of successful root canal treatment without any failures. Also, considering the literature available and other studies conducted previously we can conclude that Photodynamic Therapy can be used effectively during antimicrobial procedures of root canal system along with conventional disinfection procedure for sterilization of due to its deep penetration for bacteria’s. Hence, in future more research can be conducted related to photodynamic therapy use in dentistry.