A favourable outcome of the endodontic treatment of teeth with apical periodontitis depends on effective control of the root canal infection [1]. Chemomechanical cleaning and shaping of the root canal can greatly reduce the number of microorganisms but not completely eliminate them because of anatomical complexity and the limitation in accessing the canal system by instruments and irrigants [2,3]. The need of medication increases in those cases where an infection resists regular treatments and the therapy cannot be successfully completed owing to presence of pain and exudation [4]. Therefore one of the most important objectives of endodontic treatment is to reduce the bacterial insult to minimum, allowing host’s defence system to take over and provide a favourable environment for healing [5].
The excellent biologic and antimicrobial properties of calcium hydroxide have made this medication the choice for the intracanal dressing of infected root canals. It is bactericidal and neutralizes the remaining tissue debris in the root canal system [6].
Chlorhexidinegluconate has been used as irrigant and intracanal medicament in endodontics. Its antimicrobial effect is related to the cationic molecule binding to negatively charged bacterial cell walls, thereby altering cell’s equilibrium and causing leakage of intracellular components. However, complete eradication of the bacteria is not guaranteed with the use of these agents and a search for a medicament that can predictably disinfect the root canal continues [1].
Natural products have been used in dental and medicinal practises for thousands of years and have become more popular today. Propolis is a naturally occurring resinous substance that honey bee collect from various plants and mix it with wax flakes and their saliva in the hive. This mixture is what they use to cover hive walls, fill cracks or gaps and embalm dead invaders [7]. The chemical composition of propolis is very complex.The chemical composition of propolis varies widely due to climate, season and location. Also, the chemical formula is not stable [8].
In this study, we are evaluating propolis for its use as an intracanal medicament and comparing its antibacterial efficacy with the commonly used medicaments i.e calcium hydroxide and 2% chlorhexidine against E faecalis.
Materials and Methods
One hundred and twenty human extracted anterior single rooted teeth with patent root canals and fully developed root apices were selected for the study.
1. Preparation of dentin specimens
The model proposed by Haapasalo and Ørstavik (1987) was modified. The teeth were decoronated below CEJ and the apical parts of root were removed with the rotary diamond disc to obtain 6mm of middle of the root. Gates Glidden drill no 3 (Mani, Inc, Prime Dental Products) in a slow speed handpiece (NSK, Tokyo, Japan) was used to standardize the internal diameter of root canal. The cementum was removed with diamond cylindrical bur (Mani Dia-Burs, Prime Dental Products) to standardize the external diameter to approximately 4 mm [Table/Fig-1].The specimens were placed in an ultrasonic bath of 17% ethylenediaminetetraacetic acid for 5 min followed by 3% NaOCl for 5 min to remove organic and inorganic debris. The traces of chemicals used were removed by immersing the dentine specimens in an ultrasonic bath containing distilled water for 5 min. All the specimens were sterilized in an autoclave in 2 cycles. The first cycle was carried out at 1210C and the second cycle was with the specimens immersed in 1 mL of tryptone soya (TS) broth in individual microcentrifuge tubes.
120 study samples - 6mm of middle portion of root and theinternal diameter of root canal is standardized with gates glidden drill no 3
2. Contamination of the specimens
The test organism used for this study was E. faecalis ATCC 35550. E. faecalis was grown in tryptone soya agar for 24 h. The culture was suspended in 5 ml of TS broth and incubated for 4 h at 370C. Each dentine block was placed in pre-sterilized microcentrifuge tubes containing 1 ml of the TS broth. Fifty microlitres of the inoculums containing the E. faecalis was transferred into each of the microcentrifuge tubes. At the end of 24 h, the dentine specimens were transferred into fresh broth containing E faecalis. All the procedures were carried out under laminar flow. Purity of the culture was checked by subculturing 5 μl of the broth from the incubated dentine specimens in TS broth on tryptone soya agar plates. Contamination of the dentine specimens was carried out for a period of 21d.
3. Antimicrobial assessment
At the end of 21 d, the specimens were irrigated with 5 mL of sterile saline to remove the incubation broth. They were assigned into four groups (n = 30 dentine blocks).
Group 1, Saline (negative control); (0.9%w/v, sodium chloride injection, Marck Biosciences Ltd, Goa, India)
Group 2, Propolis; (RK’s Aroma Products, Mumbai)
Group 3, 2% CHX; (Asep- RC, Stedman pharmaceuticals Pvt Ltd, Chennai, India)
Group 4, Calcium hydroxide, (Dentpro, Ammdent, Mohali, India)
In groups 2 (Propolis solution) and 3 (2% Chlorhexidine solution), methylcellulose was added as thickening agent to make a gel formulation [3]. The gel was placed in the canal with the syringe with delivery tips. In group 4, Calcium hydroxide was mixed with saline in the ratio of 1.5:1 (w/v) to make a paste like consistency. It was placed with lentulospiral (Mani, Inc, Prime Dental Products) in the canal and condensed with blunt end of the paper point into canal.
After the placement of medicaments, the canals were sealed at both ends with paraffin wax. They were incubated in an anaerobic environment at 370C.
At the end of 1, 3, and 5 days an assessment of microbial cells was carried out with 10 specimens at each time interval. The canals were irrigated with saline to remove the medicament. Harvesting of dentin was carried out at a depth of 400 μm with Gates Glidden drill no 5 (Mani, Inc, Prime Dental Products).
The collected dentin shavings were transferred into 1 mL of sterile TS broth and incubated in an anaerobic environment at 370C for 24 h. After 24 h, the contents of each tube was serially diluted, 100μL of the broth in 100μL of sterile saline five times. Fifty microlitres of the dilution was then plated on TS agar plates and incubated for 24 h. Colonies were counted with the digital colony counter and readings were tabulated.
Statistical Analysis
The data were analysed statistically with one-way analysis of variance followed by Scheffe multiple comparison test to check the difference in bacterial inhibition between groups (p < 0.05). The paired t-test was used to check for differences in growth at different time intervals within the same group (p < 0.05).
Results
The current study showed that all 3 medicaments studied exerted antibacterial activity. The number of colony-forming units in all the experimental groups was significantly lower in comparison with the control group (Saline) on day 1, 3 and 5 (p < 0.05) as shown in [Table/Fig-2].
Mean colony counts CFU/ml for different medicaments at different time intervals at the depth of 400 μm, Colony count (x 105)
| Day 1 | Day 3 | Day5 |
|---|
| Saline | 3.36 | 3.41 | 3.33 |
| Propolis | 1.13 | 1.06 | 0.98 |
| 2% CHX | 0 | 0 | 0 |
| Ca(OH)2 | 1.65 | 1.28 | 1.27 |
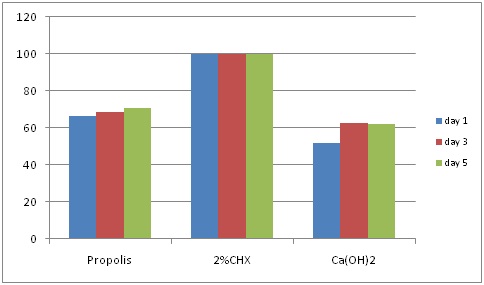
Group 3- 2% chlorhexidine gel was found to be the most effective (100%) against E. faecalis at the depth of 400 μm on all days of incubation as shown in [Table/Fig-3].
Percentage efficacy of intracanal medicaments at different time intervals
| Day 1 | Day 3 | Day5 |
|---|
| Propolis | 66.37 | 68.91 | 70.57 |
| 2% CHX | 100 | 100 | 100 |
| Ca(OH)2 | 50.89 | 62.46 | 61.86 |
Intergroup comparison between groups showed significant difference between propolis (66.37%) and Ca(OH)2 (50.89%) on day 1 (p=0.002), i.epropolis was more effective than Ca(OH)2 on day 1 but there was no significant difference in their effectiveness on day 3 (p=0.168) and day 5 (p=0.084) [Table/Fig-4].
Percentage efficacy of the medicaments at different time intervals
Discussion
The use of intracanal medications to disinfect the root canal system has been advocated to enhance the success of root canal treatment [12].
The model proposed by Haapasalo and Orstavikwas modified for this study, The presence of the cementum affected the ability of the E. faecalis cells to infect the dentinal tubules, therefore, the cementum was removed from the specimens [13,14].
Contamination period of dentin with E. faecalis was 21 d. Haapasalo and Ørstavik reported that after 3 wk of incubation of dentine discs with E. faecalis, a dense infection from the canal reached up to the depth of 300–400 μm in the dentinal tubules [14].
Calcium hydroxide is believed to have many of the properties of an ideal root canal dressing mainly due to its alkaline pH Estrela et al., [15] claimed that Ca(OH)2 by means of hydroxyl ions inhibits bacterial enzymes of the bacteria’s cytoplasmic membrane, generating the antibacterial effect.
In this study, calcium hydroxide showed minimal antimicrobial effect compared with propolis and chlorhexidine. For calcium hydroxide to act effectively as an intracanal dressing, it should ideally occupy all the pulp space and should have close contact with the microorganism. Perhaps such contact does not occur in the total root canal system, where microorganisms can be located inside the dentinal tubules. Moreover, the low solubility and diffusibility of Ca(OH)2, as well as the dentine buffering ability may make it difficult to attain an increased pH capable of eliminating bacteria located within dentinal tubules or enclosed in anatomical variations [6].
Evans et al., demonstrated that the proton pump activity of E. faecalis offers resistance to high pH of calcium hydroxide [16]. Chlorhexidinegluconate is a cationic bisguanide that seems to act by adsorbing onto the cell wall of the microorganisms and causing leakage of intracellular components. Chlorhexidine was used in gel formulation because it imparts important properties such as low toxicity to periapical tissues, viscosity that keeps the active agent in contact with the root canal walls and dentinal tubules [17].
In the present study, 2% chlorhexidine gel provided 100% inhibition of E. faecalis from day 1 to day 5. The possible reason could be the increased concentration of chlorhexidine i.e. 2% and increased diffusion of the medicament into the dentinal tubules. Basrani et al., found lower contact angle in different preparations containing chlorhexidine, enabling better diffusion into tubules [18].
Moreover, the presence of chlorhexidine adds substantivity to the formulation, due to its adsorption capacity and slow liberation of active molecules by dental tissues [6]. Another advantage is that it does not produce resistant microorganisms.
According to the results of the study, propolis has better antibacterial efficacy when compared to calcium hydroxide on day 1 but there was no significant difference between them on day 3 and day 5. Mechanisms of propolis activity against microorganisms had been explained in a number of ways. The antibacterial action can be attributed to its flavonoid contents like quercetin, galangin, pinocembrin, esters of caffeic acid, benzoic acid and cinnamic acid [19]. In addition the ultraviolet absorbing component of propolis has been shown to inhibit bacterial DNA dependant RNA polymerase [20]. It is reported that propolis prevents bacterial cell division and act on the microbial membrane or cell wall site causing functional and structural damages, similar to the action of some antibiotics [21,22].
There are no reports dealing with bacterial resistance to constituents of propolis [23]. Recent studies reported that propolis is more effective against resistant microorganisms as well as biocompatible to the periradicular tissues than existing medicaments [20].
It is difficult to contrast the results of different studies on propolis antimicrobial activity against E. faecalis due to differences in propolis formulations as well as in microbiologic methods and the time period of intracanal medication. Its composition varies with the flora of a given area, the time of collection and the inclusion of wax contaminants. This could vary the clinical effectiveness of propolis on the intracanal microflora.
Further research of these alternative medicaments is necessary before clinical application is considered.
Conclusion
This in vitro study evaluated the antimicrobial efficacy of 2% Chlorhexidine gel, Propolis and Calcium hydroxide against Enterococcus faecalis at the depth of 400μm in human root dentin at the end of 1, 3 and 5 days. Under the limitations of the study, following are the conclusions:
2% Chlorhexidine consistently demonstrated significant (100%) inhibition against E. faecalis at day 1, 3 and 5.
The antimicrobial efficacy of Propolis was greater than Ca(OH)2 at day 1, but there was no significant difference at day 3 and day 5.
Propolis can be used an effective alternative intracanal medicament.