Introduction
While acute stress responses elicits adaptation and survival by positively acting on neural, cardiovascular, immune and metabolic pathways, chronic stress deregulates those systems by switching their function to a pathophysiological mode. Although glucocorticoids and catecholamines are the two landmark hormones of stress response, several other mediators such as brain neurotrophic factor (BDNF), tissue plasminogen activator, polysialated neural cell adhesion molecule as well as pro- and anti-inflammatory cytokines and excitatory amino acids, are also involved in the adaptative biological modifications whether effective or detrimental .
In particular, stress enhances BDNF mRNA levels aimed to counteract the lowered excitability in hyppocampal neurons [1,2] following corticosteroids-related exhaustion of BDNF itself [3]. This may help explaining some apparent conflicting experimental data showing that chronic stress down-regulates BDNF synthesis [3,4] unlike others which reported the opposite [1]. Moreover, very recently it has been found that while high-novelty-seeking rats (high responders) are vulnerable to the induction of depressive-like symptoms by social defeat stress, low-novelty-seeking rats (low responders) are not and this is a further variable in BDNF gene regulation [5].
Finally, the neurotrophic factors release and regulation in the brain is decreased during aging process and this is most evident in the course of neurodegenerative diseases such as Parkinson’s and Alzheimer’s diseases [6]. As a matter of fact, it is well-established that stress is a robust factor of neuronal injury and it can bring about degenerative cellular processes in the limbic system.
On the interventional viewpoint, a great deal of interest is being dedicated to the connection between neurotrophic factors and the above diseases as well as depression. Indeed, the upregulation of BDNF, NGF, GDNF and other factors is regarded as a treatment goal of depression and neurodegenerative diseases [7]. BDNF is a small dimeric protein which regulates cellular mechanisms mediating synaptic plasticity [8] and decreased BDNF signalling can impair several brain functional aspects. Infact, impaired level of BDNF in the hippocampus is associated with altered synaptic plasticity, cognitive performances and mood-related disorders [9]. We have recently demonstrated that a quality-controlled marine nutraceuticals (a bacteriologically-free, fish-egg derived protein gel preparation, LD-1227, Caviarlieri, LabDom, Switzerland) could effectively protect experimental stress-induced hyppocampal degeneration [10] when oxidative but not neurotrophic factor were analysed. Moreover, from other recent studies some of our co-authors had also highlighted the effect of this biomarine compound on some fundamental mechanisms affecting key inflammatory molecules involved in metabolic syndrome [11]. This holds of interest when considering the novel hypothesis of the neurotrophic theory of metabolic syndrome [12].
Thus, the aim of this prospective study was to see whether LD-1227 could beneficially modulate BDNF, as measured in the serum, in otherwise healthy but work-stressed individuals.
Materials and Methods
Patients and Methods
A total of 48 men and women between the ages of 38 and 62 were recruited for this study and all gave a written informed consent to the study. Patients were selected to have an overall positive attitude towards their personal life but reporting high-demanding work activity regarded as stressful and were eligible for the study if they reported being sedentary. A sedentary adult was defined as one who has not engaged in an exercise regimen exceeding 20 min per day, 3 day per week over the previous six months [13]. Participants with a major depression/anxiety diagnosis were excluded as well as if they were excessive drinkers (>10 drinks/week) and illicit drugs users or presenting with a past or present history of cancer, fish-allergy heart disease or autoimmune disorders. Participants who were overweight (BMI>27) or poor sleepers, or suffering from sleep-apnea or attending mind-body relaxation programs were also regarded as ineligible for the study. Similarly, cases suffering at present or in the past from severe burnout (high score for emotional exhaustion or depersonalization) as assessed by a slightly modified Maslach Burnout Inventory [14] were also excluded. In our study we purposely excluded those patients with Val66Met functional polymorphism of BDNF since this has been found to be associated with an impaired release of BDNF [15], reduced hyppocampal size and decreased memory and executive function in humans [16] while, in some cases, being more common to post-stress or post-trauma depression [17]. Healthy, age/gender-matched group fulfilling the above exclusion criteria and reporting no psychological pressure of any kind, were considered our non-stressed control group [Table/Fig-1].
Occupational factors such as qualifications and assigned task, professional record, professional category, working conditions, relationship with other members of the staff, amount of work were recorded [Table/Fig-2]. All subjects participated in the study on a voluntary and informed basis. Written consent was obtained from all subjects before the study and the study conforms to the ethical guidelines of the 1975 Declaration of Helsinki. Subjects were randomized using a table of random numbers derived from a random number generating program. A single master sheet was used to randomize subjects if they were eligible for inclusion in the study following the clinical screen. The master sheet included subject number and date of randomization was used to check the process and insure no duplications of assignment. Routine blood chemistry results were within laboratory normal ranges except five patients in the treatment group and three in the placebo group who had mild hypercholesterolemia which was treated only with diet.
Subjects were divided in two group (24 patients each) and double-blindly supplemented for two month with: a) high-purity caviar-derived DNA, collagen elastin and protein extracts from sturgeon (LD-1227, Caviarlieri, 400mg marine protein/capsule, which has a protein/lipid ratio of 3.6 and the following major class of fatty acids: saturated fatty acids: 23%, monounsaturated fatty acids 33%, polyunsaturated fatty acids 34%, with a median n-6/n-3 fatty acid ratio of 2.7. In particular, the main fatty acids g/100g of total fatty acids are as follows: C22:6 5.8, C16:0 15.8, C18:1 33.7 and C18:2 24.4. LD-1227, LabDom, Switzerland) or b) placebo (same looking like capsule containing cellulose). Subjects consumed the assigned product for nine weeks, with blood samples taken before and after the supplementation period. Subjects were instructed to maintain their prior dietary regimen during participation. A third group (group C) of 25 healthy, non-stressed individuals (as for specific questionnaire and interview assessment) and who were age- and gender –matched served as control and were supplemented LD-1227, as above. During each visit, all participants confirmed the accuracy of their health screening form.
Genetic analysis of BDN F polymorphism
Genetic analysis was carried out using real time PCR and LightCycler 2.0 (Roche Diagnostics) The following SNPs were analysed: – BDNF rs6265 Ex11 Val66Met 11p13; the melting temperature for the G (Val) allele Tm: 56.94°C; for the A (Met) allele Tm: 62.83°C.
Stress questionnaire
Psychological stress was measured by the State Trait Anxiety Inventory (STAI), which is widely used for assessing state or acute anxiety [18]. STAI was completed by all participants at the entry and at the completion of the study. The STAI asks the subject to describe how he/she feels ‘right now’ by responding to 20 questions with a 4-point response format from ‘not at all’ (score 1) to ‘extremely’ (score 4) anxious. The answers are in the form of quadripartite Licker scale. Total scores range from 20 to 80, with higher scores indicating greater anxiety. This measure has been shown to have high reliability and high construct validity. The questionnaire was administered on a weekly base by telephone interview and during each schedule 1 and 2 month visit.
Sleep Quality: The Pittsburgh Sleep Quality Index (PSQI), is reported to have high internal validity and an overall reliability coefficient, and was used to examine sleep duration, sleep quality, and napping behaviour over the past month, during and after the study period [19]. In particular, the PSQI is designed to assess sleep quality during the past month and contains 19 self-rated questions from which 7 component scores are calculated and summed into a global score. Higher scores represent worse sleep quality: component scores range from 0 to 3, and global scores range from 0 to 21. The questionnaire was administered on a weekly base by telephone interview and during each schedule 1 and 2 month visit.
Psychological well-being assessment: Bond and Lader’s visual analogue scale (VAS)
Bond–lader visual analogue scales (VAS) [20], using 16 horizontal 100 mm scales were used to assess subjective mood and the effect of supplement was calculated by analyzing the following parameters: alertness, contentedness and calmness. In particular, the scales reflect three key mood factors: “alert” (alert-drowsy, attentive-dreamy, lethargic-energetic, muzzy-clearheaded, coordinated-clumsy, mentally slow-quick witted, strong-feeble, interested-bored, incompetent-proficient), “calm” (calm-excited, tense-relaxed) and “content” (contented-discontented, troubled-tranquil, happy-sad, antagonistic-friendly, withdrawn-sociable). Item scores were summed and averaged to create total scores for each respective factor with a potential range between 0 and 100, the latter value representing highly alert, calm or content. The questionnaire was administered on a weekly base by telephone interview and during each schedule 1 and 2 month visit.
Laboratory analysis
Salivary Amylase Participants were instructed not to eat or drink (except water) for 30 min prior to saliva collection and to rinse their mouth with clear water immediately before each collection. Unstimulated saliva was collected and stored at −70°C until assayed. Before assay, the saliva samples were centrifuged for a short time in an Eppendorf Microfuge to remove solid material and precipitates and salivary amylase was determined using an ELISA kit from Salimetrics (Pennsylvania, USA).
Serum BDNF measurement: Blood was collected in the morning from the antecubital vein and left at room temperature for one hour to allow clotting, followed by one hour at 4°C for platelet activation, blood was centrifuged at 4°C 2200rpm for 10 min and serum was separated and stored at -70°C until the time for BDNF measurement. Serum levels of BDNF was quantitatively measured by a commercially available sandwich ELISA (Promega BDNF EmaxH, Madison, USA), according to the instructions provided by the manufacturer, in 96-well plates using a specific human BDNF antibody. No significant cross-reactivity has been reported in prior tests using this assay. Briefly, the plates were coated with 100 μl of mouse anti-human BDNF antibody. Wells were washed three times and 100 μl of standards and samples were added in duplicate and incubated for 2 h. Wells were then washed extensively with wash buffer, 100 μl of biotinylated mouse anti-human BDNF antibody was added and incubation set for 2 h. Afterwards, 100 μl of Streptavidin-Horse Radish Peroxidase was added for 20 min. Wells were washed, 100 μl of substrate was added and incubated for 20 min and the absorbance was measured within 30 minutes in a microplate reader at 450 nm to determine BDNF concentrations according to the standard curve. These were previously designed using different concentrations (ranging from 30 to 1500 pg/ml) and were tested in duplicate on each plate. Samples were appropriately diluted (between 1:100-1:150) and BDNF concentrations were then calculated by non-linear regression from the standard curves. Measurements were repeated in duplicate and value expressed in ng/ml after correcting for sample dilution. Assays were made in duplicate on the same day, means were calculated and intra-assay variability was less than 8%. To evaluate inter-assay variability, an internal control consisting of serum obtained from one individual, frozen in multiple aliquots, was run on each plate processed. BDNF concentrations of this control sample were measured on several different days and multiple 96-well plates. This resulted to be less than 11%.
Statistical Analysis
Statistical analysis was performed using the x2 statistic or Fisher’s Exact Test for independence. Pairwise analysis was performed as appropriate. Two-tailed tests of significance were used throughout. Correlations were assessed using linear regression analysis. When necessary, log transformation was used to normalize the data, or appropriate nonparametric tests were employed. If factor time was significant, post hoc tests with Bonferroni correction were carried out to compare concentrations to baseline concentrations. Data are presented as the median given and the significance level for all tests was set at p<0.05.
Results
All participants confirmed that they had maintained a good health throughout the study and had not taken any recreational or prescription drugs, with the exception of oral contraceptive pills. The healthy, non-stressed individuals also reported unchanced life style and no main emotional even occurring during the study period.
No significant weight change was observed and, as a whole, routine blood tests were not affected by LD-1227 supplementation (data not shown) and sleep quali-quantitative assessment (PSQI) was not significantly modified by the treatment (data not shown). All subjects supplemented with LD-1227 who had tested positive as for “daytime dysfunction” question at the entry (5/24: 20% ) normalized this parameter at the end of the study period but the limited number did not enable a statistical analysis. None of the placebo treated group reporting same complain (4/24: 16%) showed any difference at the end of the study.
State Anxiety assessment (STAI-Y1)
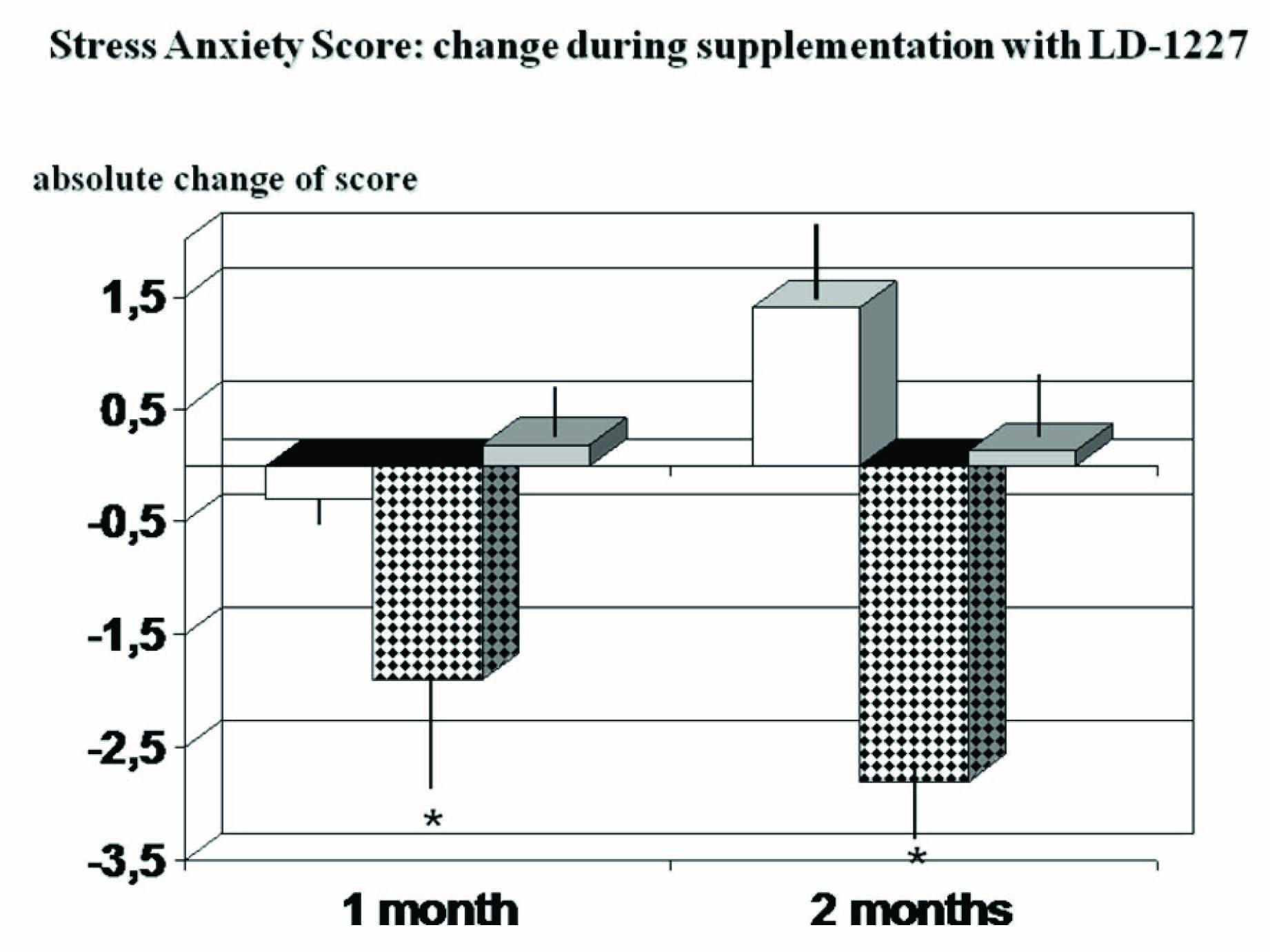
Data from the state anxiety inventory revealed that, as compared to not-stress individual, the stressed ones scores a statistically significant higher value [Table/Fig-3] (p<0.05). Placebo treatment brought about only a minor not significant trend decrease of the score during the first month check up but the overall values at the end of the study were comparable to their counterpart baseline. On the contrary, LD-1227-treated subjects showed a significant decrease of STAI score already at one month observation and such effect was maintained till the end of the study period (p<0.05). For correlation with salivary amylase, see below.
Psychological well-being assessment: Bond and Lader’s visual analogue scale (VAS).
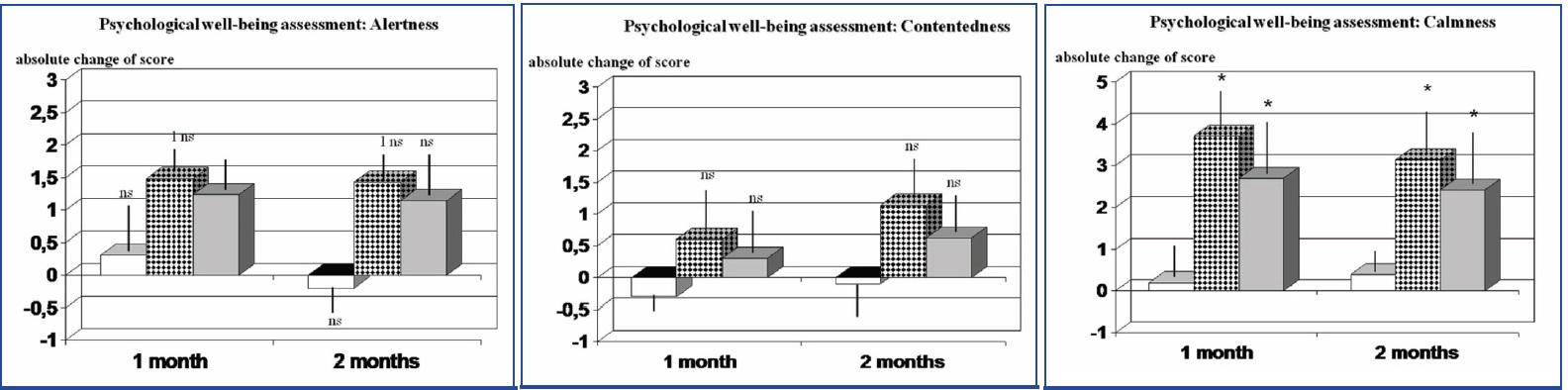
Alertness Scores of alertness increased did not show any significant difference between groups at the entry into the study not during supplementation with LD-1227 [Table/Fig-4a]. Nine patients, supplemented LD-1227, and 5 subjects of group C, reported a constantly better alertness when waking up in the morning starting after 8-10 d of supplementation and this was sustained throughout the study but the overall score during the day was not significantly better when compared to placebo. Nonetheless, no such event was reported in the placebo group. If analyzing this parameter taking into account only half-day evaluation, this phenomenon reached a significant statistical values (group C vs placebo, p<0.005, data not shown). Contentedness A not significant trend increase of this parameter was observed in group B as compared to the other groups [Table/Fig-4b] (p<0.064). This trend was not affected also when splitting the day in two different parts. Calmness LD-1227-supplemented group showed a statistically significant improvement of this parameter as compared to baseline and to the other groups (p<0.05). This phenomenon was noted by 54% of patients (13/24) at the beginning of the second week but reached a statistical significance after three weeks and remained unaltered till the end of the study period [Table/Fig-4c] (p<0.05).
Salivary Amylase
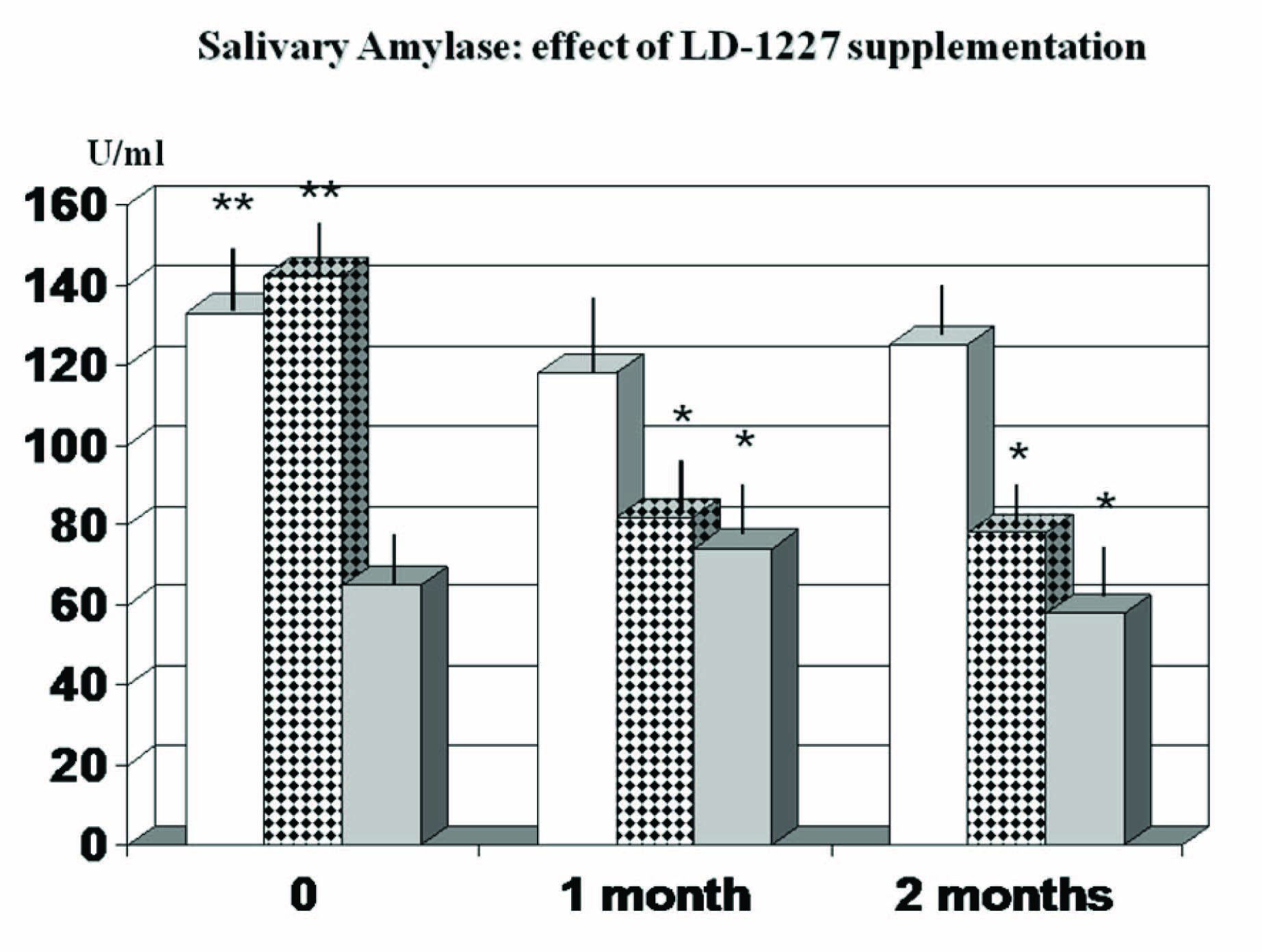
As compared to healthy, non-stressed individuals, stressed subjects showed a statistically significant increase in salivary amylase [Table/Fig-5] (p<0.01). When plotting these values against the STAI scores, it appeared a significant correlation (r: 0.63, p<0.05) in stressed subjects at baseline but not in the other groups either at baseline and when followed up during the study period (data not shown). Such significant correlation at baseline observed in stressed subjects was somehow blunted when this group was given placebo and only a positive not-significant trend correlation was noted (r: 0.42, p<0.082). This group showed also a not-significant trend decrease of this parameter during the study period (p<0.071).
Serum BDNF
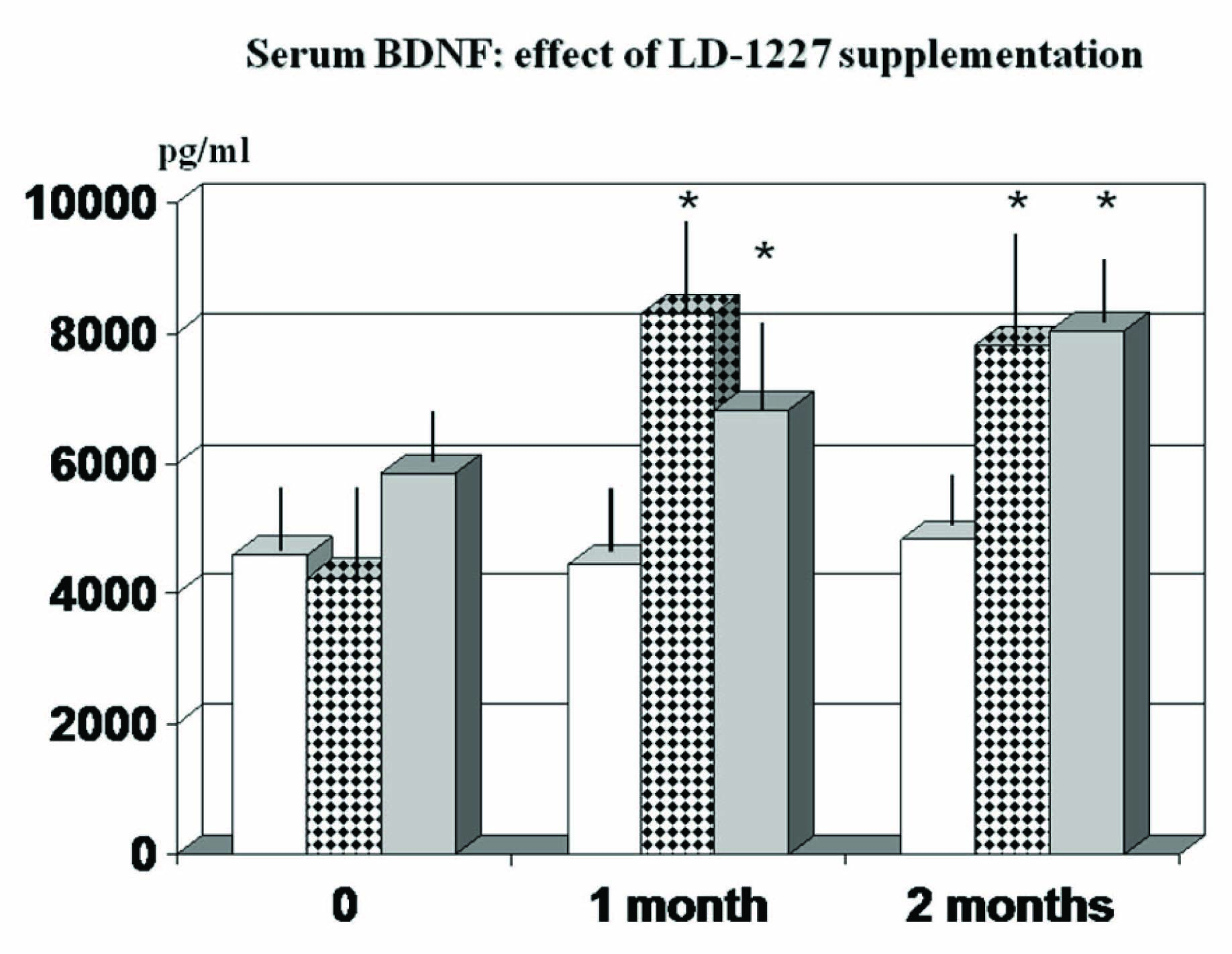
When analysing life-style pattern and biochemical values, it appeared a significant association of BDNF with smoking (r: 0.4, p<0.01, data not shown), which was included as a covariate in further analyses. As compared to non-stressed individuals, the stressed group showed a trend decrease of BDNF value but this did not reach a statistical significance. However, when the supplementation with LD-1227 brought about a significant increase of this parameter when observed at 1 and 2-month observation [Table/Fig-6] (p<0.01) and this phenomenon occurred irrespective of the stressed or non-stressed group.
Discussion
Chronic stress is known to induce increased levels of adrenal glucocorticoids and morphologic abnormalities in limbic areas [21]. Indeed the hippocampus is a main structure for spatial learning and memory and, together with the amygdala and prefrontal cortex [22] and is susceptible to stress and senescence when glucocorticoid injury produces dendritic remodeling of CA3 pyramidal neurons and a decrement in adult neurogenesis in the dentate gyrus [23]. Moreover, these alterations are related also to emotional response impairments [21] and stress represents also the major precipitating factor in mood disorders. In this context and under physiological conditions, brain-derived neurotrophic factor (BDNF), is recognized as a key component in the regulation of neurite outgrowth and survival, differentiation, and homeostatic maintenance of function in different neuronal populations and adult neuroplasticity as a whole. Serum BDNF levels mainly mirror the amount of BDNF stored and released from platelets where it has almost the same concentration than in serum [24,25] and 20- to 50-fold more represented than in plasma [26].
The occurrence of acute stress bouts supervening a chronic stress status is likely to bring about a relentless functional impairment in BDNF gene regulation, up to compromising the ability to effectively respond to challenging mental situations. Indeed, Smith et al., have shown that exposure to immobilization stress results in a dramatic decrease of BDNF concentration in the rodent hippocampus [27] and this effect is likely to take place in many other types of stress [28]. Interestingly, the induction of BDNF with increase BDNF expression in the hippocampus has been reported with different antidepressants [29] and this factor has been considered a drug target also for AD [30]. There are a number of potential mechanisms that could be advocated for the regulation of BDNF by stress such as gene transcription with a reduction of neuronal firing, since BDNF expression is dependent on activity and Ca2+- stimulated [31]. Given the as yet insurmountable methodological and pharmacokinetic issues to directly implement BDNF treatment, the identification of BDNF mimetics potentially activating the BDNF receptor or downstream targets of BDNF signalling are a matter of intensive medical research [32].
Some of our group have recently published that LD-1227 was able to beneficially modulate some key biochemical markers involved in metabolic syndrome [11]. Given that BDNF inhibits food intake and increases energy expenditure, those findings together with the current ones may be reinforced by the very recent study of Rothman et al., [33] suggesting a rationale for targeting BDNF signalling for novel therapeutic interventions in a range of metabolic and neurological disorders. Moreover, we have recently shown that this quality-controlled marine nutraceuticals could effectively protect experimental stress-induced hyppocampal degeneration [10].
This holds of interest when considering that LD-1227 contains collagen elastin, protein and a rich array of other smaller unsaturated fatty acids, and structural phospholipids and the most recent work from Hashimoto et al., points out the superiority of such multiple lipidic moieties as compared to eicosapentaenoic acid alone in improving reference memory-related learning ability or stress-handling and BDNF [34,35]. Indeed, the hypothesis that PUFAs and their metabolites beneficially interact with BDNF and other neurotrophic factors has been recently suggested [36].
Sleep loss results in higher stress vulnerability and is often found in mental disorders where BDNF may be playing a central role in this relationship [37]. None of our subjects suffered from insomnia and this may be explain the lack of a consistent effect of LD-1227. However, the albeit not significant, total improvement of the “daytime dysfunction” parameter reported by 20% of stressed individuals may be tentatively related to the significant improvement of “calmness” evaluation from those supplemented with LD-1227.
Limitations in our study were the pre-selection exclusion of individual who expressed Val66Met functional polymorphism of BDNF, as well as the lack of oxidative stress tests and their possible correlation with BDNF as elsewhere suggested [38] as well as a specific evaluation of the influence of physical activity synergies [39]. Finally, different age-related hormonal balance in our patients could together with possible gut microbiota interplay, as recently suggested [40,41], are worth being considered in further ongoing studies with LD-1227
Flow chart of patients selection for the study
| Flow Chart of Patients selection for the study |
| Male | Female |
| Male/female | 41 | 26 |
| Excluded for unfavourable BDNF-SNP profile | 4 | 3 |
| Excluded for occasional use of sleeping tablets and unavailability to stop them | 4 | 3 |
| Excluded for prior depressive episodes requiring treatment | 1 | 1 |
| Excluded for prior burn out event | 0 | 1 |
| Excluded for impending or ongoing divorce | 1 | 1 |
| Drop out | 0 | 0 |
Subjects demographics (stressed individuals)
| Male/female | 31/17 |
| Mean age (age range) | 47 (39-65) |
| Occupation |
| Busy professionals: Laywers (4), Surgeons (plastic, orthophedics, Gynecology) (9) | 13 |
| Businessmen (high rank bank employee, financial promoters) | 9 |
| Shop managers | 18 |
| Marital status |
| married | 21 |
| single (with partner) | 18 (16) |
| divorced (with partner) | 9 (8) |
| STAI (at entry) | 43.1 ± 0.7 |
| PSQI (at entry)
| 4.0 ± 1.0 |
State Anxiety Score: absolute change during LD-1227 supplem-entation., * p<0.05 vs stressed placebo-supplemented controls
Psychological well-being assessment during LD-1227 supplementation. Effect on absolute score change for: Alertness [Table/Fig-4a], Contentedness.,and Calmness When analyzing this parameter taking into account only the first half-day evaluation, this phenomenon reached a significant statistical values (group C vs placebo, p<0.005, see text) ns: not sstatistically significant; * p<0.05 vs stressed placebo-supplemented subjects
Effect of LD-1227 supplementation on salivary amylase, * p<0.05 vs stressed placebo-supplemented controls; ** p<0.01 vs healthy not stressed control subjects
Effect of LD-1227 supplementation on serum BDNF during 2 months observation study., * p<0.05 vs own baseline values and vs stressed placebo-supplemented subjects
Conclusion
Nonetheless, taken overall, from the present study it appears that there is a beneficial effects of LD-1227 on measures of psychological well-being and related anxiety in healthy and healthy-stressed individuals as well as salivary stress markers and concomitant increase of serum level of BDNF. This may suggest longer term studies in view of a neuroprotective strategy against stress-induced neurodegeneration.
The study, trial n. 5/2014, was carried out without any authors having any conflict of interest and supported by ReGenera association for Aging Intervention, a no profit organization. No author was employed or consulting for the laboratory manufacturing the marine nutraceuticals.
[1]. O Oztan, C Aydin, C Isgor, Chronic variable physical stress during the peripubertal-juvenile period causes differential depressive and anxiogenic effects in the novelty-seeking phenotype: functional implications for hippocampal and amygdalar brain-derived neurotrophic factor and the mossy fibre plasticityNeuroscience 2011 192:334-44. [Google Scholar]
[2]. F Marmigère, L Givalois, F Rage, S Arancibia, L Tapia-Arancibia, Rapid induction of BDNF expression in the hippocampus during immobilization stress challenge in adult ratsHippocampus 2003 13:646-55. [Google Scholar]
[3]. LT Yi, J Li, D Geng, BB Liu, Y Fu, JQ Tu, Essential oil of Perilla frutescens-induced change in hippocampal expression of brain-derived neurotrophic factor in chronic unpredictable mild stress in miceJ Ethnopharmacol 2013 147:245-53. [Google Scholar]
[4]. AV Paska, T Zupanc, P Pregelj, The role of brain-derived neurotrophic factor in the pathophysiology of suicidal behaviorPsychiatr Danub 2013 25:341-44. [Google Scholar]
[5]. L Zhao, JL Wang, R Liu, XX Li, Zhang Li JF, Neuroprotective, anti-amyloidogenic and neurotrophic effects of apigenin in an Alzheimer’s disease mouse modelMolecules 2013 18:9949-65. [Google Scholar]
[6]. S Amor, LA Peferoen, DY Vogel, M Breur, P van der Valk, D Breur, JM van Noort, Inflammation in neurodegenerative diseases-an updateImmunology 2014 142(1):151-66. [Google Scholar]
[7]. A Martocchia, M Curto, S Scaccianoce, F Comite, D Xenos, C Nasca, Effects of escitalopram on serum BDNF levels in elderly patients with depression: a preliminary reportAging Clin Exp Res 2014 [Google Scholar]
[8]. A Autry, L Monteggia, Brain-derived neurotrophic factor and neuropsychiatric disordersPharmacol Rev 2012 64:238-58. [Google Scholar]
[9]. R Lipsky, A Marini, Brain-derived neurotrophic factor in neuronal survival and behavior-related plasticityAnn NY Acad Sci 2007 122:130-43. [Google Scholar]
[10]. F Marotta, DH Chui, H Yadav, A Lorenzetti, G Celep, S Jain, Effective properties of a sturgeon-based bioactive compound on stress-induced hippocampal degeneration and on in vitro neurogenesisJ Biol Regul Homeost Agents 2012 26:327-35. [Google Scholar]
[11]. F Marotta, A Lorenzetti, R Catanzaro, N Zerbinati, S Jain, U Solimene, A sturgeon-derived bioactive compound beneficially modulates nuclear receptors controlling metabolic functions in patients with metabolic syndromeActa Biomed 2013 84:53-60. [Google Scholar]
[12]. MG Hristova, Metabolic syndrome - From the neurotrophic hypothesis to a theoryMed Hypotheses 2013 [Google Scholar]
[13]. M Södergren, Lifestyle predictors of healthy ageing in menMaturitas 2013 75:113-17. [Google Scholar]
[14]. AM Bouman, H Te Brake, J Hoogstraten, Significant effects due to rephrasing the Maslach Burnout Inventory’s personal accomplishment itemsPsychol Rep 2002 91:825-26. [Google Scholar]
[15]. MF Egan, M Kojima, JH Callicott, TE Goldberg, BS Kolachana, A Bertolino, E Zaitsev, The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hyppocampal functionCell 2003 112:257-69. [Google Scholar]
[16]. T Frodl, C Schüle, G Schmitt, C Born, T Baghai, P Zill, Association of the brain-derived neurotrophic factor Val66Met polymorphism with reduced hippocampal volumes in major depressionArch Gen Psych 2007 64:410-16. [Google Scholar]
[17]. JM Gatt, CB Nemeroff, C Dobson-Stone, RH Paul, RA Bryant, RH Schofield, Interactions between BDNF Val66 Met polymorphism and early life stress predict brain and arousal pathways to syndromal depression and anxietyMol Psych 2009 14:681-95. [Google Scholar]
[18]. J González-Cabrera, M Fernández-Prada, C Iribar-Ibabe, JM Peinado, Acute and chronic stress increase salivary cortisol: a study in the real-life setting of a national examination undertaken by medical graduatesStress 2014 17:149-56. [Google Scholar]
[19]. DJ Buysse, CF Reynolds, TH Monk, CC Hoch, AL Yeager, DJ Kupfer, Quantification of subjective sleep quality in healthy elderly men and women using the Pittsburgh Sleep Quality Index (PSQI)Sleep 1991 14:331-38. [Google Scholar]
[20]. A Bond, M Lader, The use of analogue scales in rating subjective feelingsBr J Psychol 1974 47:211-18. [Google Scholar]
[21]. Chattarji McEwen, Molecular mechanisms of neuroplasticity and pharmacological implications: the example of tianeptine Eur Neuropsychopharmacol 2004 14:S497-502. [Google Scholar]
[22]. KM Christian, H Song, GL Ming, Functions and Dysfunctions of Adult Hippocampal NeurogenesisAnnu Rev Neurosci 2013 [Google Scholar]
[23]. F Darcet, I Mendez-David, L Tritschler, AM Gardier, JP Guilloux, DJ David, Learning and memory impairments in a neuroendocrine mouse model of anxiety/depressionFront Behav Neurosci 2014 8:136 [Google Scholar]
[24]. H Fujimura, CA Altar, R Chen, T Nakamura, T Nakahashi, J Kambayashi, Brain-derived neurotrophic factor is stored in human platelets and released by agonist stimulationThromb Haemost 2002 87:728-34. [Google Scholar]
[25]. M Lommatzsch, D Zingler, K Schuhbaeck, K Schloetcke, C Zingler, P Schuff-Werner, The impact of age, weight and gender on BDNF levels in human platelets and plasma Neurobiol Aging 2005 26:115-23. [Google Scholar]
[26]. JG Lee, BS Shin, YS You, JE Kim, SW Yoon, DW Jeon, JH Baek, SW Park, YH Kim, Decreased serum brain-derived neurotrophic factor levels in elderly korean with dementiaPsychiatry Investig 2009 6:299-309. [Google Scholar]
[27]. MA Smith, S Makino, R Kvetnansky, RM Post, Stress alters the express of brain-derived neurotrophic factor and neurotrophin-3 mRNAs in the hippocampusJ Neurosci 1995 15:1768-77. [Google Scholar]
[28]. N Tsankova, O Berton, W Renthal, A Kumar, R Neve, EJ Nestler, Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action.Nat Neurosci 2006 9:465-66. [Google Scholar]
[29]. D Munno, S Sterpone, S Fania, F Cappellin, G Mengozzi, M Saroldi, Plasma brain derived neurotrophic factor levels and neuropsychological aspects of depressed patients treated with paroxetinePanminerva Med 2013 55:377-84. [Google Scholar]
[30]. PS Aisen, Serum brain-derived neurotrophic factor and the risk for dementia.JAMA 2014 311:1684-85. [Google Scholar]
[31]. M Metsis, T Timmusk, E Arenas, H Persson, Differential usage of multiple BDNF promoters in the rat brain following neuronal activationProc Natl Acad Sci U S A 1993 90:8802 [Google Scholar]
[32]. SJ Allen, JJ Watson, DKA Shoemark, NU Patel, GDNF, NGF and BDNF as therapeutic options for neurodegenerationPharmacol Ther 2013 138:155-75. [Google Scholar]
[33]. SM Rothman, KJ Griffioen, R Wan, MP Mattson, Brain-derived neurotrophic factor as a regulator of systemic and brain energy metabolism and cardiovascular healthAnn NY Acad Sci 2012 1264:49-63. [Google Scholar]
[34]. M Hashimoto, T Inoue, M Katakura, Y Tanabe, S Hossain, S Tsuchikura, Prescription n-3 fatty acids, but not eicosapentaenoic acid alone, improve reference memory-related learning ability by increasing brain-derived neurotrophic factor levels in SHR.Cg-Lepr(cp)/NDmcr rats, a metabolic syndrome modelNeurochem Res 2013 38:2124-35. [Google Scholar]
[35]. LT Yi, J Li, D Geng, BB Liu, Y Fu, JQ Tu, Essential oil of Perilla frutescens-induced change in hippocampal expression of brain-derived neurotrophic factor in chronic unpredictable mild stress in miceJ Ethnopharmacol 2013 147:245-53. [Google Scholar]
[36]. U Vetrivel, SB Ravichandran, K Kuppan, J Mohanlal, UN Das, A Narayanasamy, Agonistic effect of polyunsaturated fatty acids (PUFAs) and its metabolites on brain-derived neurotrophic factor (BDNF) through molecular docking simulationLipids Health Dis 2012 11:109 [Google Scholar]
[37]. M Giese, E Unternaehrer, S Brand, P Calabrese, E Holsboer-Trachsler, A Eckert, The interplay of stress and sleep impacts BDNF levelPLoS One 2013 8(10):e76050 [Google Scholar]
[38]. S Jain, BD Banerjee, RS Ahmed, VK Arora, PK Mediratta, Possible role of oxidative stress and brain derived neurotrophic factor in triazophos induced cognitive impairment in ratsNeurochem Res 2013 38:2136-47. [Google Scholar]
[39]. Y García-Mesa, H Pareja-Galeano, V Bonet-Costa, S Revilla, MC Gómez-Cabrera, J Gambini, Physical exercise neuroprotects ovariectomized 3xTg-AD mice through BDNF mechanismsPsychoneuroendocrinology 2014 45:154-66. [Google Scholar]
[40]. WY Tzeng, LH Chen, CG Cherng, YN Tsai, L Yu, Sex differences and the modulating effects of gonadal hormones on basal and the stressor-decreased newly proliferative cells and neuroblasts in dentate gyrusPsychoneuroendocrinology 2014 42:24-37. [Google Scholar]
[41]. E Schéle, L Grahnemo, F Anesten, A Hallén, F Bäckhed, JO Jansson, The gut microbiota reduces leptin sensitivity and the expression of the obesity suppressing neuropeptides proglucagon (Gcg) and brain-derived neurotrophic factor (Bdnf) in the central nervous systemEndocrinology 2013 [Google Scholar]