Morphometry, Asymmetry and Variations of the Sylvian Fissure and Sulci Bordering and Within the Pars Triangularis and Pars Operculum: An Autopsy Study
Olufemi Emmanuel Idowu1, Sunday Soyemi2, Kazeem Atobatele3
1 Neurosurgery Division, Department of Surgery (Neurosurgery Unit), Lagos State University College of Medicine and Lagos State University, Teaching Hospital, Ikeja, Lagos, Nigeria.
2 Forensic Medicine, Department of Pathology, Lagos State University College of Medicine and Lagos State University Teaching Hospital, Ikeja, Lagos, Nigeria.
3 Neurosurgery Division, Department of Surgery (Neurosurgery Unit), Lagos State University College of Medicine and Lagos State University Teaching Hospital, Ikeja, Lagos, Nigeria.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Idowu, Olufemi Emmanuel, Neurosurgery Division, Department of Surgery, Lagos State University College of Medicine, Ikeja, Lagos, Nigeria. Phone : +234 08023 451 369, E-mail : oeidowu412@yahoo.com
Objective: Speech and Language, one of the most lateralized of all cerebral functions is located within the pars opercularis (PO) and pars triangularis (PT). There is also inter-hemispheric variability of the sulcal contours bordering these areas. The study was undertaken to note the morphometry, asymmetry and variations of the Sylvian fissure (SF), and the sulci bordering and within the PO and PT.
Materials and Methods: An adult autopsy cadaveric study was carried. The measurements made amongst others, included fronto-occipital cerebral length, cerebral width, Sylvian fissure length, and anterior Sylvian point (ASP) to inferior Rolandic point distance. The PT and PO were also studied.
Results: Sixty-two adult cadaveric hemispheres were studied. The SF length on the right (mean=84.3mm, median=88mm) was significantly shorter than that on the left (mean=89.4mm, median=92.0mm) (p=0.037). The anterior ascending and anterior horizontal rami of the SF arose from the ASP and either divides at this point (43 hemispheres, 69.4%) or have a common short stem before separating distally giving a Y-shape configuration. The triangularis sulcus was noted in 49 hemispheres (79%) while the diagonal sulcus was noted in 26 hemispheres (41.9%).
Conclusion: The left SF was significantly longer than the right and both were positively correlated. The presence of the triangularis sulcus was not dependent on the side (p=0.348) or gender (0.622) unlike the diagonal sulcus was side dependent (p= 0.000).
Diagonal sulcus, Pars opercularis, Pars triangularis, Sylvian fissure, Triangular sulcus
Introduction
Clinically and with respect to functional neuroimaging, speech and language are one of the most lateralized of all cerebral functions [1]. Their cortical areas are also some of the most asymmetrical in the brain [2]. These areas are located within the pars opercularis (PO) and pars triangularis (PT) in the frontal lobe. They are bounded anatomically by the anterior horizontal ramus of the Sylvian fissure (SF), anterior ascending ramus of the SF. These sulcal delineations are used to delimit the PO and PT from surrounding cortex [3]. They are also used as anatomical boundaries to estimate gyral volume of these regions [4–6].
It is well known that the different cerebral regional structures are closely related to their functions; however the borders between these zones are sometimes not as distinct as they are taught. Despite the classic anatomical books documentations, it is not unusual for difficulty to ensue while trying to recognize undoubtedly the various cerebral sulci due to asymmetry and variations. For over a century great variability in the configuration of cerebral sulci, not only among different brains, but between the two hemispheres of individual brains has been noted [7]. The surface appearance of the cerebral sulci varies greatly from brain to brain, and between hemispheres of the same individual brain [8,9]. Notwithstanding these variations, the deep cortical structures are remarkably consistent [8].
The cerebral sulci of fissure serve as landmarks to choose surgical passageway to reach the deeply located parts of the brain or skull base [10]. The sulci or fissure used predictably will depend on the surgical approach, which is also dependent on the intracranial lesion. The SF is a commonly used surgical corridor.
The PT and PO may or may not have a triangularis sulcus or diagonal sulcus respectively. The presence of the diagonal sulcus of Eberstaller in the PO is more frequently observed in the left hemisphere relative to the right [4,10,11]. There is individual variability in the sulcal bordering of the PT and PO, which gives rise to great variability in size, surface area and volume of the PO and PT [12]. Leftward volume asymmetry of the PO and PT may exist in the human brain given the clinical functional asymmetries observed with respect to language expression. Contrariwise, post-mortem and magnetic resonance imaging (MRI) studies have failed to consistently identify such a volumetric asymmetry [12]. An emergent question is whether the asymmetry in function is linked to the asymmetry in gross anatomy.
The areas of anterior perisylvian speech-language regions which include the PT, PO and the adjoining SF are of considerable significance in clinical neurology. Intra-operatively, precise localization and appreciation of the anterior perisylvian speech-language areas to prevent new deficits arising from brain dissection and retraction is crucial, more so in the light of intra-operative brain shifts [13].The useful and practical intraoperative frameless imaging devices besides being very expensive and not available in many neurosurgical centres, cannot substitute the basic neuroanatomical familiarity that should be part and parcel of a neurosurgeon’s armamentarium, ditto for a neuropathologist. The surgeon’s knowledge of the structure and a better appreciation of the range of their variation in the human brain, is vital to interpret functional imaging studies and during intra-operative dissection [14,15].
The aim of this study is to document the morphometric measurements, evaluates for asymmetry and variation of the SF and sulci bordering and within the PT and PO.
Materials and Methods
A total of 62 cerebral hemispheres from the 31 autopsy cadavers were examined. The human brains were obtained at autopsy from the Lagos State University Teaching Hospital, Ikeja, Lagos, Nigeria in line with our University hospital guiding protocols and regulations.
The specimens were taken from adult (18 y and above) individuals of either gender. The brains of 12 men and 19 women were examined. Individuals with preserved anatomical brain structures were included in the study. Specimens from individuals with neurological causes of death were excluded.
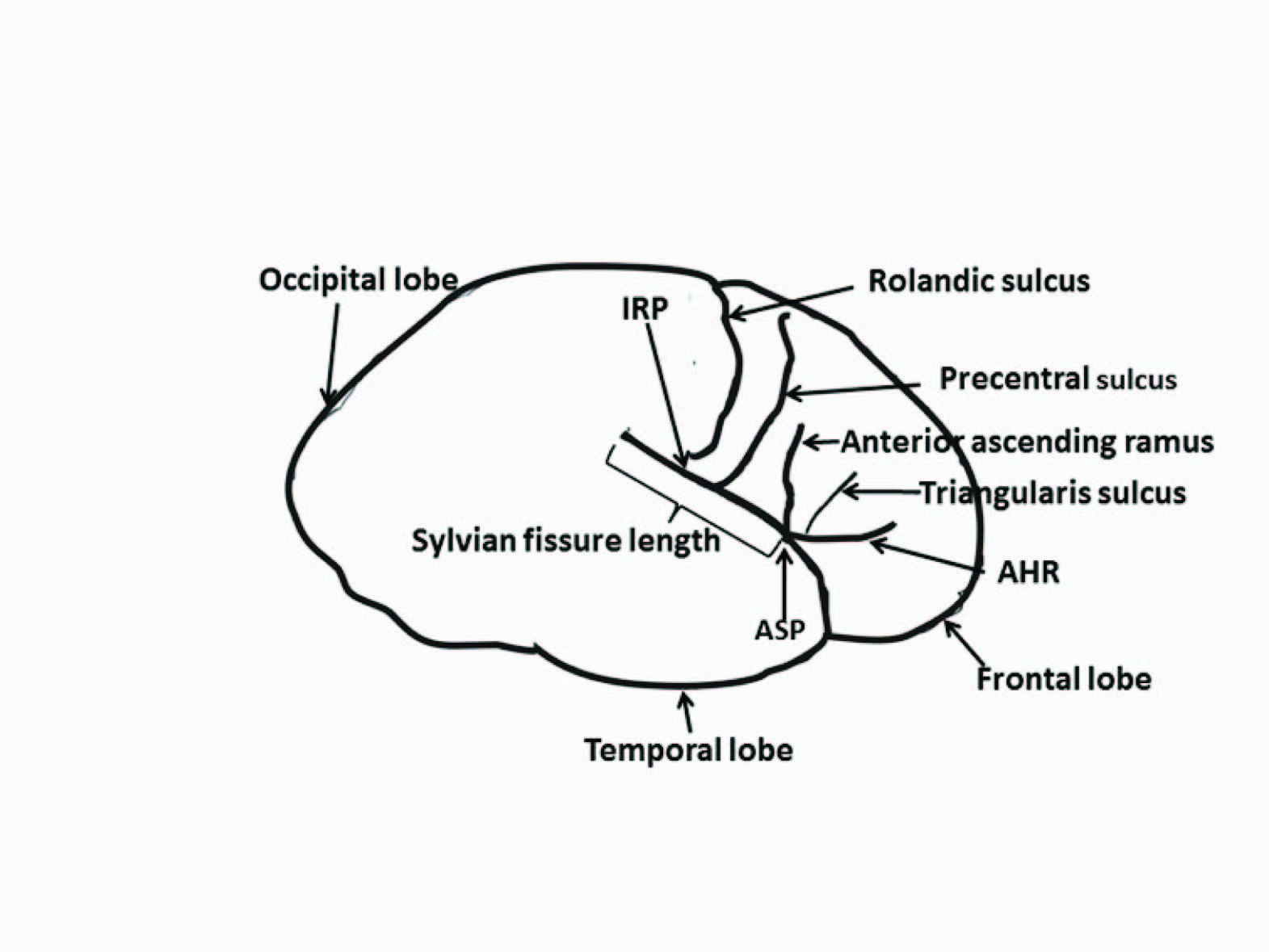
Each specimen was identified by its autopsy case number and information pertaining to gender and age were unknown to the investigators until the analysis was complete. The various sulci were identified in each hemisphere according to standard anatomical criteria [5]. Identification of the interested sulci was established by consensus between two of the authors and was based on previously established criteria. The intersection of the caudal extension of the Rolandic sulcus and the SF was referred to as inferior Rolandic point (IRP). Measurements were subsequently made while keeping the arachnoid matter intact. The measurements made were fronto-occipital cerebral length, cerebral width (at the level of the IRP to the midline), SF length (from the anterior Sylvian point to where the SF separates into ascending and descending posterior rami), anterior Sylvian point (ASP) to IRP distance, and ASP to precentral sulcus in each cerebral hemisphere [Table/Fig-1]. The ASP is defined in this study as the most anterior part of SF. The sulcus (if present) located within the PT and or PO was also evaluated. Encountered variations were examined noted.
Illustration of the lateral surface of the brain examined, *AHR- Anterior horizontal ramus of the Sylvian fissure, ASP- Anterior Sylvian point, Inferior Rolandic point (IRP), SF- Sylvian Fissure,
Statistical Analysis
Statistical analysis of the data was performed using t-test (means comparison) and chi-square (or Fischer’s exact test) as appropriate. McNemar test was use to examine the difference between the proportions. The association between continuous variables was investigated by means of Pearson’s correlation coefficient. Significance was judged at p<0.05 level. The data was computed and analysed using the Statistical Package for Social Sciences version 17 statistical software (SPSS Inc., Chicago, IL, USA).
The procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional or regional) and with the Helsinki Declaration of 1975 that was revised in 2000.
Results
The brains of 12 men and 19 women were examined. They had a mean age of 48.5 y (median-44.5y, range 26-75y). The mean fronto-occipital length and width are as depicted in [Table/Fig-2]. The cerebral width mean was 67.1mm (median 67.0mm) on the right and 69.9mm (median 70mm) on the left. The cerebral width/length ratio was 0.37 on the right and 0.38 on the left. The fronto-occipital length (p=0.965), cerebral width (p=0.165), ASP to IRP (p=0.407) and ASP to precentral sulcus (p=0.724) dimensions were not significantly different on both sides. They were however, positively correlated.
The length of the Sylvian fissure and anterior sylvian point to the inferior Rolandic point
| MRI pattern | Right SF | Left SF | Right ASP-IRP | Left ASP-IRP | Right FO | Left FO |
|---|
| Median (mm) | 88.0 | 92.0 | 48.0 | 48.0 | 185.0 | 184.0 |
| Mean (mm) | 84.3 | 89.4 | 47.5 | 48.9 | 183.8 | 183.8 |
| Minimum (mm) | 30.0 | 37.00 | 22.0 | 20.0 | 165.0 | 165.0 |
| Maximum(mm) | 120.0 | 122.0 | 82.0 | 90.0 | 200.0 | 202.0 |
| SD | 17.923 | 17.506 | 10.869 | 13.894 | 10.314 | 10.527 |
| SEM | 3.219 | 3.144 | 1.952 | 2.495 | 1.852 | 1.890 |
| Percentiles |
| 3rd | 30.0 | 37.0 | 22.0 | 20.0 | 165.0 | 165 |
| 25th | 79.0 | 83.0 | 42.0 | 38.0 | 172.0 | 175 |
| 50th | 88.0 | 92.0 | 48.0 | 48.0 | 185.0 | 184 |
| 75th | 95.0 | 100.0 | 52.0 | 55.0 | 192.0 | 191 |
| 95th | 109.2 | 116.0 | 70.0 | 80.4 | 200.0 | 200.8 |
| 97th | 120.0 | 122.0 | 82.0 | 90.0 | 200.0 | 202.0 |
*ASP- Anterior Sylvian point; FO- Fronto-occipital; IRP- Inferior Rolandic point; SD-Standard deviation; SEM- Standard error of mean SF- Sylvian fissure
The mean SF length was 86.9mm (30-122mm). On the right it was 84.3mm (median=88mm, range 30-120mm), and 89.4mm (median=92.0mm, range 37-122mm,) on the left [Table/Fig-2]; while it was 83.5mm (median=82.5mm, range 30-120mm) on the right and 87.8mm (median=91mm, range 65-110mm) on the left for males, and on the right and left for females it was 84.8mm (range 30-102mm, median=90mm) and 90.4mm (range 37-122mm, median=95mm) respectively. The left SF was however significantly longer than the right and both were positively correlated [Table/Fig-3]. The SF length asymmetry was not dependent on gender (p=0.574).
Cerebral width and length of the Fronto-occipital, cerebral Sylvian fissure, anterior Sylvian point (ASP) to inferior Rolandic point lengths and ASP to the precentral sulcus
| Length | Median | Mean | SD | SEM | Student’s t (p-value) | r (p-value) |
|---|
| Fronto-occipital (mm) |
| Right | 185.0 | 183.5 | 10.31 | 1.85 | | |
| Left | 184.0 | 183.8 | 10.53 | 1.89 | -0.044 (0.965) | 0.923 (0.000) |
| Cerebral width (mm) |
| Right | 67.0 | 67.1 | 8.53 | 1.53 | | |
| Left | 70.0 | 69.9 | 11.45 | 2.06 | -1.423 (0.165) | 0.440 (0.013) |
| Sylvian fissure (mm) |
| Right | 88.0 | 84.3 | 17.92 | 3.22 | | |
| Left | 92.0 | 89.4 | 17.51 | 3.14 | -2.179 (0.037) | 0.734 (0.000) |
| ASP-IRP (mm) |
| Right | 48.0 | 47.5 | 10.87 | 1.95 | | |
| Left | 48.0 | 48.9 | 13.89 | 2.50 | -0.842 (0.407) | 0.764 (0.000) |
| ASP-PC sulcus (mm) |
| Right | 30.0 | 28.7 | 7.63 | 1.37 | | |
| Left | 28.0 | 29.9 | 10.29 | 1.85 | -0.724 (0.475) | 0.508 (0.004) |
*ASP- Anterior Sylvian point; IRP- Inferior Rolandic point; PC- Precentral; SD-Standard deviation; SEM- Standard error of mean
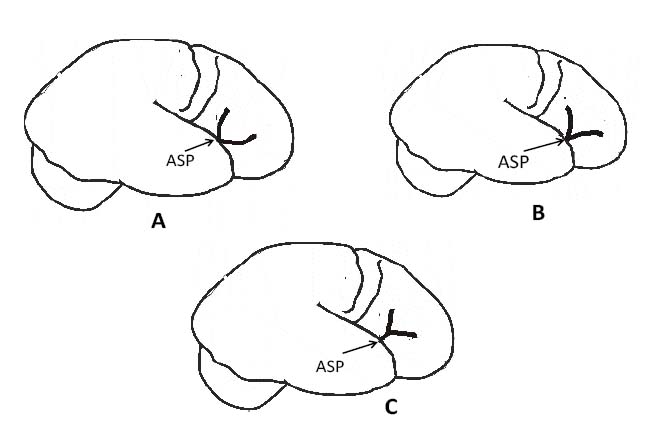
The anterior ascending and anterior horizontal rami of the SF arose from the ASP and either divides at this point (43 hemispheres, 69.4%) giving a U- or V-shaped anterior ascending and anterior horizontal rami of the SF, or have a common stem (19 hemispheres, 30.6%) which separates distally giving a Y-shape anterior ascending and anterior horizontal rami of the SF configuration [Table/Fig-4&5]. There was no significant side difference with respect to the site of separation of the anterior ascending and anterior horizontal rami of the SF (p=0.409). The anterior ascending and anterior horizontal rami of the SF was U-shaped in 37.1% (23/62), V-shaped in 32.3% (20/62). The right hemispheres had a U-shaped and V-shaped configuration of the anterior ascending and anterior horizontal rami of the SF in 12/31 and 11/31 hemispheres respectively; this was 11/31 and 9/31 on the left respectively. A common stem of the anterior ascending and anterior horizontal rami of the SF that divided distally (Y-shaped) was 8/31(25.8%) on the right and 11 (35.5%) on the left hemisphere. The shape of the anterior ascending and anterior horizontal rami of the SF was not side dependent (p=0.698).
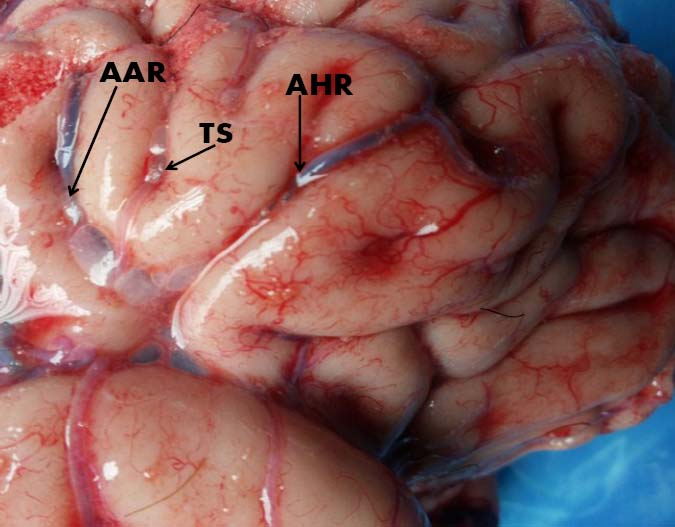
Y-shape anterior ascending and anterior horizontal rami of the SF configuration, *AAR- Anterior ascending ramus of the Sylvian fissure, AHR- Anterior horizontal ramus of the Sylvian fissure, TS- Triangularis sulcus
The various configuration of the anterior ascending and anterior horizontal rami of the Sylvian Fissure, A) U-shaped, B) V-shaped and C) Y-shaped., * ASP- Anterior Sylvian point
The triangularis sulcus was noted in 49 hemispheres (79.0%) [Table/Fig-6] while the diagonal sulcus of Eberstaller was noted in 28 hemispheres (45.2%). The presence of the triangularis sulcus was not dependent on the side (p=0.348) neither was it gender related (p=0.622). However, the presence of the diagonal sulcus was usually present on the left (p=0.000) but no difference in the male or female hemispheres (p=0.928).
Triangularis sulcus presence, p=0.348
| Side | Present | Absent | Total |
|---|
| Right | 26 | 5 | 31 |
| Left | 23 | 8 | 31 |
| Total | 49 | 13 | 62 |
Discussion
Brain asymmetry has been observed in animals and humans in terms of structure, function and behaviour. This lateralization is thought to reflect evolutionary, hereditary, developmental, experiential and pathological factors [16].
Although the cerebral sulci and gyri are easily identified with magnetic resonance imaging, it may be difficult to precisely locate them intra-operatively. The extensiveness of anatomic variations, the necessity to approach to the sulci from a small aperture during minimally invasive procedures further underscores the importance of knowledge of the cerebral anatomy of the SF, and sulci demarcating the PO and the PT [17].
The SF, a main surgical corridor and the most identifiable feature of the superolateral brain surface divides both the frontal and parietal lobes from the temporal lobe below. We noted that this was significantly longer on the left.This is similar to previous studies [18].The SF is known to be one of the most asymmetric structures of the human brain. In post-mortem brains of 35 schizophrenic patients and 33 matched non psychiatric control subjects, Falkai et al., noted that the SF showed a significantly reduced length of the left SF (-16%, p < 0.0001) compared to the control subjects, while the right SF length was unchanged. The SF asymmetry (left/right ratio) was more reduced in male schizophrenics (-24%, p less than 0.001) than in female patients (-16%, p less than 0.03) [19]. This finding is consistent with several post-mortem and MRI studies showing left temporal lobe pathology or opioid abuse [20,21].
The anterior horizontal and anterior ascending rami of the SF usually arise in close proximity to the ASP which is a small widening of the subarachnoid space that divides the SF in its main anterior and posterior rami. The anterior ascending rami of the SF, which marks the division between the PO and PT, is a deep vertical ramus rising up from the SF into the inferior frontal gyrus; it is located anterior to the diagonal sulcus, when present, and infrequently may be submerged within the inferior precentral sulcus [3,4]. Infrequently the anterior ascending rami of the SF may be absent. Ono et al., detected it in 86.66% (13/15) of the right hemispheres and in 93.33% of the (14/15) left hemispheres. In our study we found the anterior ascending rami in all the hemispheres. The anterior horizontal ramus of the SF, which demarcates the PT from the more ventrally located pars orbitalis, appears like a continuation of the SF in the lateral-orbital frontal lobe, located approximately where the temporal lobe ends.
The PT is demarcated posteriorly from the PO by the anterior ascending ramus of the SF, dorsally from the middle frontal gyrus by the inferior frontal sulcus, and rostro-ventrally from the pars orbitalis by the anterior horizontal ramus of the SF. The anterior horizontal and anterior ascending rami of the SF had three major configurations. The most common we noted was the U- and V-shaped, both accounting for almost 70%. This is at variant to Ayberk et al., who noted Y-shaped configuration in 39.3% (11/28) and 28.6% (8/28) each with V-shaped and U-shaped configuration [17].
Within the PO there is occasionally a sulcus present called the diagonal sulcus. This is visibly distinct from the anterior ascending ramus of the SF as a clear sulcus lying on the inferior frontal gyrus between the inferior precentral sulcus and the anterior ascending ramus of the SF. It divides the anterior portion of the opercular part into two triangular portions that are positioned inversely to each other [22]. The morphology of this sulcus does not constitute a uniform appearance, as it occasionally merges with the anterior ascending ramus of the SF or extends from the inferior precentral sulcus or inferior frontal sulcus. In some cases the diagonal sulcus does not merge with any of the surrounding sulci and abuts with the SF. Keller et al, described great variation in the morphology of the diagonal sulci and significant inter-hemispheric differences in the presence of the diagonal sulcus within the PO [12]. We noted the diagonal sulcus in 28 hemispheres (45.2%) and a significant higher presence on the left (p=0.000) in concordance with Keller et al.
Conclusion
The left SF was significantly longer than the right and both were positively correlated. The presence of the triangularis sulci is commonly more encountered compared to the diagonal sulci; its presence in the cerebral hemisphere is not dependent on the side (p=0.348) or gender (0.622) unlike the diagonal sulci which is usually more common on the left (p= 0.000).
*ASP- Anterior Sylvian point; FO- Fronto-occipital; IRP- Inferior Rolandic point; SD-Standard deviation; SEM- Standard error of mean SF- Sylvian fissure
*ASP- Anterior Sylvian point; IRP- Inferior Rolandic point; PC- Precentral; SD-Standard deviation; SEM- Standard error of mean
[1]. Knaus TA, Corey DM, Bollich AM, Lemen LC, Foundas AL, Anatomical asymmetries of anterior perisylvian speech-language regionsCortex 2007 43:499-510. [Google Scholar]
[2]. Greve DN, Van der Haegen L, Cai Q, Stufflebeam S, Sabuncu MR, Fischl B, A surface-based analysis of language lateralization and cortical asymmetryJ Cogn Neurosci 2013 25:1477-92. [Google Scholar]
[3]. Petrides M, Broca’s area in the human and nonhuman primate brainIn Broca’s region (edsGrodzinsky Y, Amunts K) 2006 New YorkOxford University Press:31-46. [Google Scholar]
[4]. Tomaiuolo F, MacDonald JD, Caramanos Z, Posner G, Chiavaras M, Evans AC, Morphology, morphometry and probability mapping of the pars opercularis of the inferior frontal gyrus: an in vivo MRI analysisEur J Neurosci 1999 11:3033-46. [Google Scholar]
[5]. Albanese E, Merlo A, Albanese A, Gomez E, Anterior speech region. Asymmetry and weight-surface correlationArch Neurol 1989 46:307-10. [Google Scholar]
[6]. Falzi G, Perrone P, Vignolo LA, Right-left asymmetry in anterior speech regionArch Neurol 1982 39:239-40. [Google Scholar]
[7]. Symington J, Crymble PT, The central fissure of the cerebrumJ Anat Physiol 1913 48:321-39. [Google Scholar]
[8]. White LE, Andrews TJ, Hulette C, Richards A, Groelle M, Paydarfar J, Structure of the human sensorimotor system. I: Lateral symmetryCereb Cortex 1997 7:18-30. [Google Scholar]
[9]. Gonul Y, Songur A, Uzun I, Uygur R, Alkoc OA, Caglar V, Morphometry, asymmetry and variations of cerebral sulci on superolateral surface of cerebrum in autopsy casesSurg Radiol Anat 2013 36:651-61. [Google Scholar]
[10]. Ono M, Kubik S, Abernathy CD, Atlas of the cerebral sulci 1990 New YorkGeorg Thieme Verlag:62-74. [Google Scholar]
[11]. Galaburda AM, Broca’s region: anatomic remarks made a century after the death of its discovererRev Neurol (Paris) 1980 136:609-16. [Google Scholar]
[12]. Keller SS, Highley JR, Garcia-Finana M, Sluming V, Rezaie R, Roberts N, Sulcal variability, stereological measurement and asymmetry of Broca’s area on MR imagesJ Anat 2007 211:534-55. [Google Scholar]
[13]. Figueiredo EG, Deshmukh P, Zabramski JM, Preul MC, Crawford NR, Spetzler RF, The pterional-transsylvian approach: An analytical studyNeurosurgery 2006 59:ONS263-69. [Google Scholar]
[14]. Rumeau C, Tzourio N, Murayama N, Peretti-Viton P, Levrier O, Joliot M, Location of hand function in the sensorimotor cortex: MR and functional correlationAm J Neuroradiol 1994 15:567-72. [Google Scholar]
[15]. Sanes JN, Donoghue JP, Thangaraj V, Edelman RR, Warach S, Shared neural substrates controlling hand movements in human motor cortexScience 1995 268:1775-77. [Google Scholar]
[16]. Toga AW, Thompson PM, Mapping brain asymmetryNature Reviews Neuroscience 2003 4:37-48. [Google Scholar]
[17]. Ayberk G, Yagli OE, Comert A, Esmer AF, Canturk N, Tekdemir I, Anatomic relationship between the anterior sylvian point and the pars TriangularisClin. Anat 2012 25:429-36. [Google Scholar]
[18]. Foundas AL, Faulhaber JR, Kulynych JJ, Browning CA, Weinberger DR, Hemispheric and sex-linked differences in Sylvian fissure morphology: a quantitative approach using volumetric magnetic resonance imagingNeuropsychiatry Neuropsychol Behav Neurol 1999 12:1-10. [Google Scholar]
[19]. Falkai P, Bogerts B, Greve B, Pfeiffer U, Machus B, Fölsch-Reetz B, Loss of sylvian fissure asymmetry in schizophrenia. A quantitative post mortem studySchizophr Res 1992 7:23-32. [Google Scholar]
[20]. Knaus TA, Tager-Flusberg H, Foundas AL, Sylvian fissure and parietal anatomy in children with autism spectrum disorderBehav Neurol 2012 25:327-39. [Google Scholar]
[21]. Kivisaari R, Rapeli P, Van Leemput K, Kähkönen S, Puuskari V, Jokela O, Cerebral measurements and their correlation with the onset age and the duration of opioid abuseJ Opioid Manag 2010 6:423-29. [Google Scholar]
[22]. Ribas GC, The cerebral sulci and gyriNeurosurg Focus 2010 28(2):2 [Google Scholar]