Periodontitis is a chronic inflammatory disease that causes destruction of the tooth-attachment apparatus. Regenerative periodontal therapy aims to predictably restore the structure and function of the lost tissues [1]. It is well known that periodontal regeneration is predictable to some extent in intrabony and grade II furcation defects, whereas horizontal bone loss is least predictable type of periodontal defects. Horizontal bone loss is the most common problem confronting the clinician but has received scant attention. In a recent prevalence study (2011), it was reported that vertical bone loss with a prevalence of 7.8% received 96.8% treatment options, whereas horizontal bone loss, with an overwhelming prevalence of 92.2%, received only 3.2 % treatment modalities [2].
Several treatment modalities have been attempted throughout the years including, composite grafts, Guided Tissue Regeneration (GTR) membranes and a combination of GTR and grafts materials [3–7]. Enamel Matrix Protein (EMP) and recombinant human Bone Morphogenic Protein (rhBMP) have also been tested for the treatment of horizontal defects [8–12]. Supracrestal graft application in conjunction with pocket elimination surgery has often resulted in periodontal deformities and root exposure. The outcomes of these treatments have been different with varying degrees of improvement for different techniques. With the advent of biologic approaches and other biomaterials, there is a renewed interest in the field of horizontal periodontal regeneration.
rhBMP application has been particularly studied extensively in animal models and have shown positive results with respect to bone regeneration [9–12]. EMP treatment showed better clinical improvements as compared to the conventional flap debridement of horizontal type of bone loss over a period of 8 months [8]. One of the drawbacks of EMP application is that it is expensive which cannot be routinely used. A combination of GTR technique with a sustained growth factor delivery release provides a valid treatment option for the treatment of challenging periodontal osseous defects.
Platelet-derived growth factor (PDGF) is regarded as one of the principal wound healing growth factor [13]. Cell Surface Receptor for PDGF is present on gingiva, periodontal ligament and cementum. PDGF is chemo-attractant for osteoblast, gingival and periodonal ligament fibroblast and it stimulates osteoblasts, fibroblasts and periodontal ligament cells [14].
Though the use of growth factors have shown tremendous promise in periodontal regenerative approaches, the routine use of these growth factors in everyday clinical practice has not been possible due to the non availability of an ideal carrier [1].
A recent innovation in the field of medicine/dentistry is the development of autologous Platelet Rich Fibrin (PRF) as a growth factor delivery system. Platelet rich fibrin is a second generation platelet concentrate developed by Choukroun et al., [15]. The combined properties of fibrin, platelets, leucocytes, growth factors and cytokines makes platelet rich fibrin a healing biomaterial with tremendous potential for bone and soft tissue regeneration [16]. Several studies have evaluated the effectiveness of PRF in intrabony and mandibular grade II defects and have found a positive clinical and radiographic outcome [17–21]. The routine use of such an inexpensive, autologous growth factor delivery system certainly offers an attractive option for the treatment of horizontal defects.
To the best of our knowledge there are no published data with regard to the use of PRF in periodontal regenerative approaches in the management of horizontal defects. The aim of this interventional clinical trial was to assess the clinical effectiveness of PRF to bring about periodontal regeneration in horizontal defects by comparing it with conventional open flap debridement.
Materials and Methods
This controlled clinical trial with a split mouth design was conducted in the Department of Periodontics from July 2011 to September 2012.The study consisted of three groups; an experimental group I which was treated by placement of PRF gel following open flap debridement (OFD+PRF) and an experimental group II which was treated by placement of PRF gel followed by placement of PRF membrane (OFD+PRF+PRFmembrane). The control group was treated by open flap debridement (OFD) alone. Clinical and radiographic parameters were re-evaluated after nine months.
Sample Size
The ideal sample size to assure adequate power for this clinical trial was calculated following the method described by Y H Chan [22].The formulae for calculating the sample size in a clinical trial where the primary outcome of interest is the mean difference in an outcome variable between two treatment groups is given as m (size per group) = c + 1/ δ2
where δ = I μ2 - μ1 I/ σ
μ2, μ1 are the means of the two treatment groups
σ is the common standard deviation
c = 7.95 for 80% power and 10.5 for 90% power
The value I μ2 - μ1 I represents the difference in primary outcome variable between the two treatment groups.
The radiographic parameter from a previous study by Yilmaz et al., using EMP for treating horizontal defects was used as a reference to calculate sample size [8].
It was determined that 15 defects per group would be necessary to provide 80% power with an α of 0.05.
Prior to initiating this study, the patients were informed of the purpose and design of this clinical trial and were required to sign an informed consent. The study design and consent form were approved by the Institutional ethics committee, in accordance with the Helsinki Declaration of 1975 that was revised in 2000.
The study group consisted of 15 patients with a mean age of 36.30±6.44y (range 28 to 44 y). There were nine female and six male patients in the study group.
Subject Selection
The criteria for inclusion of subjects in this study were individuals who had moderate to severe periodontitis with horizontal defects with a probing pocket depth of more than 4 mm. Radiographs of sites with suspected horizontal bone loss were analysed for the pattern of bone detruction. Horizontal bone loss pattern was confirmed when the crest of the bone was parallel with a line between the Cemento Enamel Junction (CEJ) of two adjacent teeth. Sites with a distance of 4 mm or more from the CEJ to alveolar crest were included in the study. Patients with history of allergies to drugs and the use of antibiotics within previous six months were excluded. Patients, with aggressive periodontitis, with known systemic illness and taking any medications known to affect the outcomes of periodontal therapy, pregnancy/lactation and a history of smoking were excluded.
Presurgical Therapy
Each patient was given careful instructions on proper oral hygiene measures prior to the surgery. Full mouth scaling and root planing procedures were performed under local anaesthesia. Periodontal evaluation was performed six to eight weeks following phase I therapy to confirm the suitability of the sites for this study. The sites were divided into case and control groups at the time of periodontal surgery.
Clinical Parameters
A single masked examiner (NA) performed the clinical examination at baseline and nine months after the surgical procedure. The clinical parameters assessed were Probing pocket depth (measured with William’s graduated periodontal probe), Recession/enlargement, Clinical attachment level.
Radiographic Examination
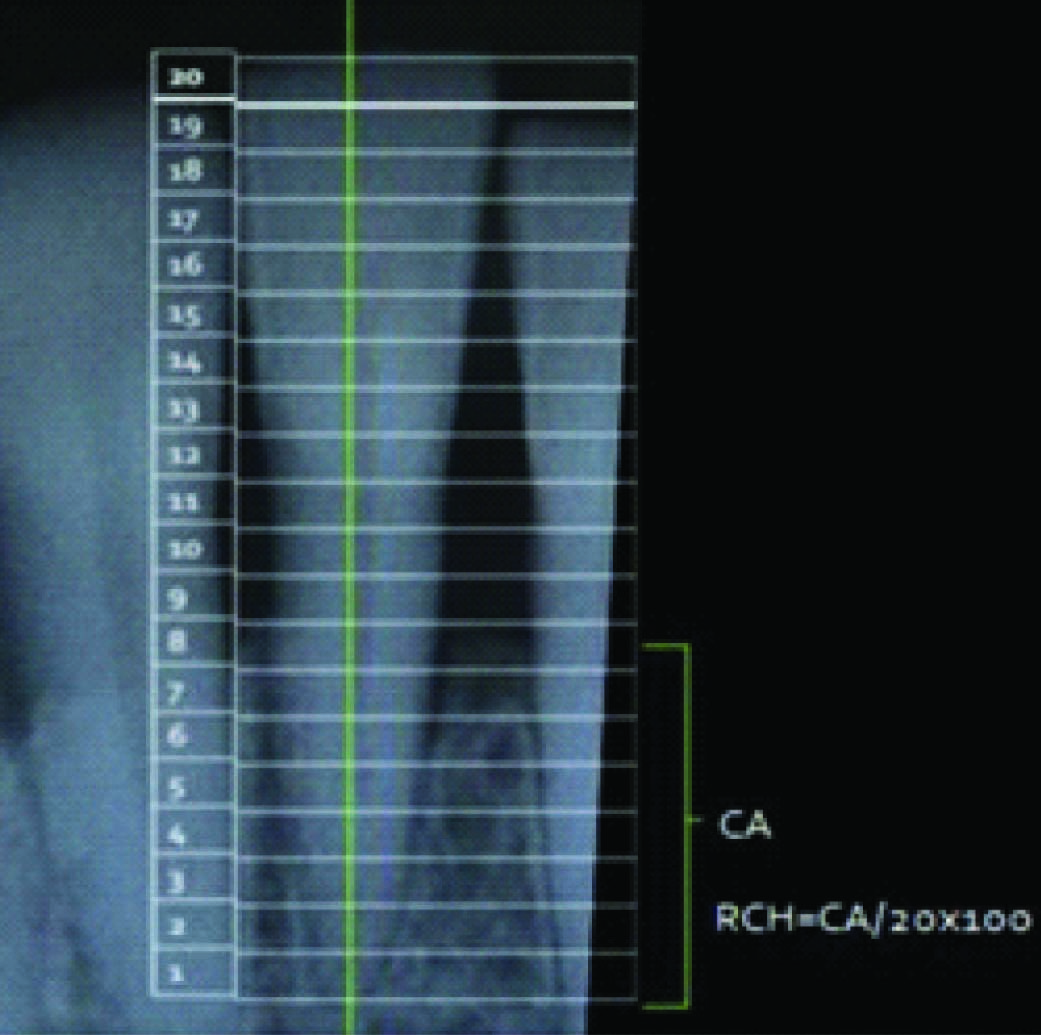
Standardized reproducible radiographs using a standardized paralleling cone technique with positioning aids were taken at each treated test and control site at baseline and nine months after surgery. All radiographs were scanned and the images were stored in a computer for assessment. The radiographic image was rotated until the long axis of the tooth was parallel to a vertical reference line [Table/Fig-1]. The distance between the radiographic crown and radiographic apex was divided into 20 equal increments with a Schei ruler [23,24]. The Schei ruler was digitally constructed using a software† and the ruler was superimposed over the radiographic image. The level of the alveolar crest was determined according to the number of increments away from the radiographic apex, which was otherwise the crest to apex distance (CA).The Relative Crest Height (RCH) to the tooth was calculated and expressed in percentages.
Radiographic assessment of bone levels using Schei ruler., CA = Crest to Apex distance, RCH = Relative Crest Height,
Surgical Procedures
A total of 45 sites in 15 patients were selected randomly for this study. A total of 3 interdental sites were taken for assessment from each patient, comprising of one site for experimental group I, one site for experimental group II and one site for control. All periodontal surgical procedures were performed by a single operator (GS). Standard surgical procedures for the test and control sites were performed as follows. After local anaesthesia, crevicular incisions were made and full-thickness mucoperiosteal flaps were elevated. The periodontal surgical procedure fully exposed the horizontal defects and preserved the marginal gingiva and interdental tissue. Meticulous defect debridement and root planing were carried out to remove subgingival plaque, calculus, inflammatory granulation tissue, and pocket epithelium. Decortication was done prior to placement of PRF to facilitate bone regeneration. The surgical sites were irrigated with normal saline and care was taken to keep the area free of saliva.
Preparation of Platelet Rich Fibrin
Blood collection and preparation of PRF was delayed until adequately exposing the bone defects so that the prepared PRF could be used instantaneously without the need for any storage.
Ten ml blood was drawn from each patient by venipuncture of the right antecubital vein. Blood was collected in sterile glass test tubes without any anticoagulants and immediately centrifuged on a table top centrifuge‡ at 3000 rpm for 10 min. This resulted in the separation of three basic fractions because of differential densities: the bottom red blood cells (RBCs), middle PRF, and the top layer of platelet-poor plasma (PPP). PPP was aspirated and discarded and the PRF gel was separated from underlying RBC layer by the use of sterile stainless steel scissors.
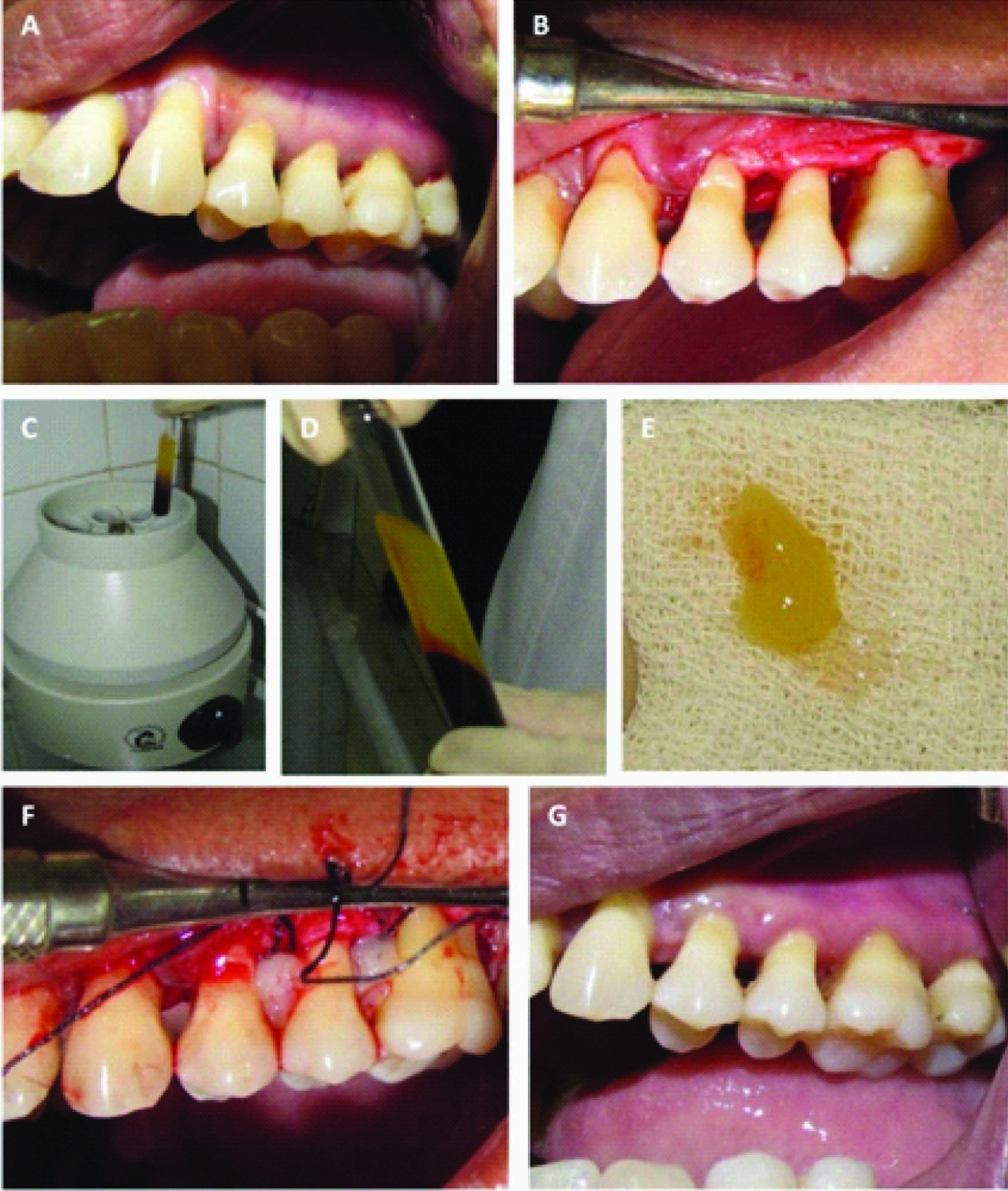
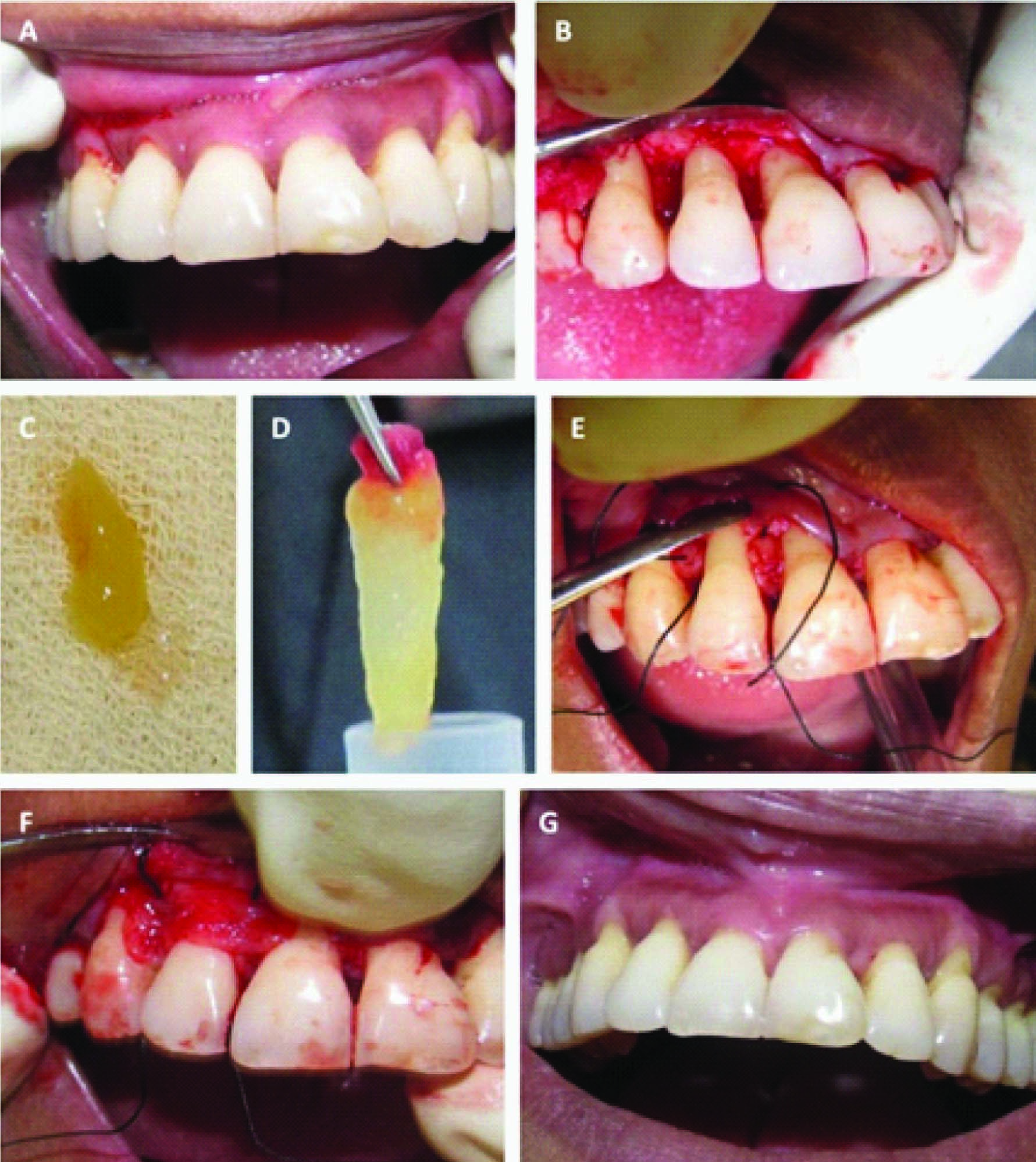
The PRF gel was immediately placed into the horizontal osseous defects in the experimental group I [Table/Fig-2A-G]. In experimental group II, PRF membrane (PRF membrane was prepared by pressing the PRF clot between two pieces of surgical gauze) was placed over the PRF gel and surgical flaps were repositioned to their presurgical level and sutured with 4-0 silk sutures§ achieving primary closure [Table/Fig-3A-G]. Periodontal packs (Coe Pack)II were placed to cover the surgical areas. Control sites were treated in every way similar to case sites except for the preparation and placement of PRF [Table/Fig-4A-D].
Clinical Photographs, Experimental Group 1 (PRF Group), A. Surgical site; B. Defects Exposed; C,D,E. Preparation of PRF gel; F. PRF gel placed between 24 & 25, 25 & 26; G. Surgical site after 9 months
Clinical photoraphs, Experimental Group II (PRF gel plus PRF membrane group); A. Surgical Site; B. Defects Exposed; C,D. Preparation of PRF gel & PRF membrance; E. PRF gel placed between 11 & 12, 12 & 13; F. PFG membrance placed over the PRF gel; G. Surgical site after 9 months
Clinical photographs, Control Group (Open Flap Debridement group); A. Surgical Site; B. Defects exposed between 43, 44, 45 & 46; C. Sutures placed; D. Surgical site after 9 months.

Postsurgical Care
Postoperative care included systemic administration of amoxicillin, 500 mg, every 8 hours for five days, paracetamol 500 mg every eight hours for three days and 0.2% chlorhexidine digluconate rinse three times daily for six weeks. Sutures were removed one week postsurgery.
Maintenance Phase
Supragingival professional tooth cleaning was performed weekly for the first 6 weeks post surgery and thereafter the patients were recalled once a month up to nine months postsurgery for oral hygiene reinforcement and prophylaxis. Clinical and radiographic assessment of the surgical sites was done after nine months [Table/Fig-5,6].
Pre and Post operative Radiograph.; A. Baselne radiograph; B. 9 months post operative radiograph

Pre and Post operative Radiograph with Schei ruler.; A. Baseline radiograph; B. 9 months post operative radiograph

Statistical Analysis
The statistical analysis was performed using commercially available software¶. All the parameters were assessed at baseline and after nine months which included Pocket Depth, Clinical Attachment level, Gingival Recession and Relative Crest Height. All the parameters were compared at baseline between groups using Kruskal Wallis Test. The mean changes at baseline and after nine months within each group were compared using Wilcoxon Signed Ranks Test. The mean changes for each parameter between groups were compared using Kruskal Wallis Test.
Results
All 15 patients completed the study and the periodontal defects in the experimental and control groups healed uneventfully. Flap dehiscence or infections were not detected in any patients.
At baseline, no statistically significant differences were detected between experimental and control sites with respect to Pocket depth (PD), Clinical attachment level (CAL), Gingival Recession (REC) and Relative bone Crest Height (RCH) [Table/Fig-7]. Mean values for clinical and radiological parameters at baseline and nine months are reported in [Table/Fig-8], and comparisons of mean changes between the groups are reported in [Table/Fig-9].
Comparison of Baseline clinical and radiographic Parameters between Groups (Kruskal Wallis test)
| Parameter | PD(mm) | CAL(mm) | REC(mm) | RCH(%) |
|---|
| Control | 4.3±0.41 | 4.23±1.47 | 0.73±0.75 | 51.33±10.6 |
| Experimental Group I | 4.33±0.69 | 4.83±1.11 | 0.96±0.51 | 50.33±10.25 |
| Experimental Group II | 4.43±0.59 | 4.4±1.25 | 0.6±0.47 | 52.0±9.22 |
| p-value | .437 | .493 | .243 | .88 |
PD: Probing Depth, CAL: Clinical Attachement Level, REC: Gingival Recession, RCH: Relative Crest Height
Comparison of Baseline and Final clinical and radiographic parameters: All Groups (Wilcoxon Signed Ranks Test)
| Parameter | PD(mm) | CAL(mm) | REC(mm) | RCH(%) |
|---|
| Control |
| Baseline | 4.3±0.41 | 4.23±1.47 | 0.73±0.75 | 51.33±10.6 |
| Final | 3.2±0.41 | 3.367±1.32 | 0.83±0.74 | 50.33±10.43 |
| Mean Changes | 1.1±0.38* | 0.86±0.58* | 0.1±0.2 | -1±2.8 |
| p value | 0.000 | .02 | .083 | .18 |
| Experimental Group I |
| Baseline | 4.33±0.69 | 4.83±1.11 | 0.96±0.51 | 50.33±10.25 |
| Final | 2.6±0.63 | 3.26±1.19 | 1.03±0.69 | 51.33±9.72 |
| Mean Changes | 1.73±0.53* | 1.56±0.62* | 0.06±0.25 | 1±4.7 |
| p value | .001 | .001 | .317 | .429 |
| Experimental Group II |
| Baseline | 4.43±0.59 | 4.4±1.25 | 0.6±0.47 | 52.0±9.22 |
| Final | 2.73±.45 | 2.7±1.14 | 0.63±.48 | 52.33±8.63 |
| Mean Changes | 1.7±0.45* | 1.7±0.52* | 0.03±0.12 | 0.33±2.28 |
| p value | .001 | .001 | .317 | .564 |
*denotes significant difference
Comparison of Mean Changes obtained for clinical and radiographic parameters between the Groups (Kruskal Wallis test)
| Parameter | PD(mm) | CAL (mm) | REC(mm) | RCH(%) |
|---|
| Control | 1.1±0.38 | 0.86±0.58 | 0.1±0.2 | -1±2.8 |
| Experimental Group I | 1.73±0.53* | 1.56±0.62* | 0.06±0.25 | 1±4.7 |
| Experimental Group II | 1.7±0.45* | 1.7±0.52* | 0.03±0.12 | 0.33.±2.28 |
| p-value | .001 | .001 | .452 | .175 |
*denotes significant difference
Re-evaluation at nine months revealed that all three treatment modalities resulted in a significant changes in PD (1.1 ±0.38 mm in control, 1.73±0.53 mm in group I, 1.7±0.45 mm in group II )(p<0.05) and CAL (0.86±0.58 mm in control, 1.56±0.62 mm in group I, 1.7±0.52 in group II) (p<0.05) compared to baseline. The magnitude of changes were higher for both the experimental groups compared to control (p<0.05) and there was no significance difference between the experimental groups (p>0.05). The changes in levels of REC (0.1±0.2 mm in control, 0.06±0.25 in group I, 0.03±0.12 mm in group II) and radiographic bone levels RCH (-1±2.8 mm in control, 1±4.7 mm in group I, 0.33±2.28 mm in group II) at baseline and nine months postsurgery showed no significant differences in all groups (p>0.05).The negative value for RCH in control group implies resorption of alveolar bone postoperatively compared to baseline.
Discussion
This interventional controlled clinical trial therefore aimed at assessing the clinical effectiveness of PRF gel and to compare its added benefits in the form of a membrane as a regenerative approach in periodontal horizontal defects, and compare both these modes of treatment to conventional open flap debridement.
A split mouth design was chosen as these permits a better assessment of how the same host responds to two different treatment modalities. Patient blinding regarding the type of therapy was not performed in this trial because the experimental sites required procedures of blood collection and preparation PRF. However the investigator performing clinical and radiographic evaluations were masked of the treatment group as to which the study site belonged.
Platelet rich fibrin alone was used in the experimental group I as a structural support for the overlying flap margins which was displaced slightly coronally while suturing in order to create space for bone regeneration. PRF membrane was additionally placed over the PRF gel in experimental group II to act as mechanical barrier to create a space around the defects that might permit periodontal regeneration and to prevent the epithelial cells from migrating into the defect area. PRF in gel and membrane form was expected to act as a source of growth factors thereby enhancing the healing process. Decortication was done with the help of the sharp end of curette prior to facilitate bone regeneration. It was performed with the intention to expose cancellous bone thereby enhancing the healing process by promoting bleeding and allowing progenitor cells and blood vessels to reach a bone-grafted site more readily [25,26]. Hou et al., had performed decortication in the management of infrabony defects along with Atrisorb membranes and achieved positive results [27].
The results of this clinical trial indicate a positive effect for the use of PRF in the management of horizontal periodontal defects in terms of improvement in clinical parameters. A number of studies have examined the effectiveness of PRF in intrabony defects and grade II furcation defects and have found positive clinical and radiographic outcomes [17–21]. The use of platelet rich fibrin matrix provides a convenient approach by which the presence of both these factors can be expected at the surgical sites which aids in endogenous periodontal regeneration.
The positive effects of PRF may broadly be attributed to the contents of the PRF clot namely fibrin, platelets, leucocytes, growth factors and cytokines [28]. Among the growth factors contained in the platelet rich fibrin clot PDGF, IGF and TGF-b play the most important roles. PDGF-α and β receptors are expressed in regenerating periodontal soft and hard tissues. More importantly, in vivo application of PDGF alone or in combination with insulin-like growth factor-1 results in partial repair of periodontal tissues [29]. In the present study we observed an improvement in radiographic bone crestal levels in the experimental groups although not statistically significant. PDGF has been shown to have a significant regenerative impact on periodontal ligament cells and osteoblasts [30]. PRF stimulates human bone mesenchymal cell proliferation and differentiation. So the actions of growth factors and leucocytes contained in the PRF could have contributed to the marginal improvement in radiographic bone levels observed in the present study [31].
Our results were compared to the clinical outcomes from previous studies aimed at horizontal regeneration [4,5,7,8]. Our findings were similar to the clinical and radiographic findings observed by Yilmaz et al., in the experimental group where EMP was used to treat horizontal defects [8].
One major disadvantage of PRF membrane is its lack of rigidity, because of which the membrane tends to collapse over the bone and root surface which may have limited the space which is necessary for clot maturation. In other studies Teflon membrane was used by Stahl et al., and ePTFE membrane by Kotschy et al., in horizontal defects to separate the gingival flap from the underlying bone and root surface and to provide the space which is necessary for clot stabilization [4,5]. However, the disadvantage of Teflon and ePTFE membrane is the need for second surgery for their removal. PRF membrane on the other hand is autologous in nature and is gradually resorbed over time. Kassolis et al., used Demineralized freeze-dried bone allografts (DFDBA) in particle, strut, and laminar forms in combination with GTR to provide structural support for the retention of DFDBA particles supracrestally [7]. Similarly in our study, the experimental group II sites utilized PRF gel to support the PRF membrane which separated the gingival flap from contact with the bone surface. However, the extent of space maintenance by PRF gel tends to be less as the liquid is slowly lost from the gel.
Limitations of the study included assessment of periodontal regeneration using clinical and radiographic parameters as done in most clinical studies. True periodontal regeneration can only be assessed in histological sections which could not be done in the present study due to ethical concerns. The present study design used standardized intraoral radiographs to assess changes in bone crest heights. Use of advanced radiographic techniques such as digital subtraction radiography would have enabled better precision in assessing new bone formation.
Conclusion
Within the limitations of the study it can be concluded that:
Use of PRF significantly improved the clinical parameters in terms of Probing Depth and Clinical Attachment Level, but there was no significant radiographic improvement in the bone levels at nine months postsurgery.
Horizontal defect being the most prevalent form of periodontal defect demands more attention by researchers and the use of autologous growth factor delivery system in the form of PRF offers a new dimension in their management.
† Microsoft Powerpoint 2007
‡ Almicro” Instruments, India
§ Ethicon, Johnson and Johnson Ltd., Somerville, NJ, USA
ι ι Coe-Pak, GC America Inc., Chicago, IL, USA
¶ SPSS 16, SPSS Inc., Chicago, IL, USA
PD: Probing Depth, CAL: Clinical Attachement Level, REC: Gingival Recession, RCH: Relative Crest Height
*denotes significant difference
*denotes significant difference