Periodontitis is a chronic inflammatory disease caused by infection with gram negative bacteria & characterized by gingival inflammation & alveolar bone destruction. It is characterized by increased expression of various cytokines and other inflammatory mediators resulting in extensive osteoclast formation and bone loss [1,2]. These cytokines affect bone remodeling and play a vital role in both the physiological and pathological regulation of bone [2,3].
Alveolar bone is constantly remodelled through the synthesis of bone matrix by osteoblast & resorption of bone by osteoclasts [1]. Bone is the specialized connective tissue comprising of various cells, collagenous and inorganic matrix. The level of bone mass which is essential to execute various functions is maintained by a balanced act of bone formation and bone resorption. This process is highly coordinated and regulated by two specialized cells, osteoblasts the bone forming cells and osteoclasts the bone resorbing cells. These cells are controlled by various hormones, inflammatory mediators, cytokines and growth factors. Discovery of the osteoprotegrin (OPG); receptor activated kappa (RANK); receptor activated kappa B ligand (RANKL) system have unravelled a long standing unexplained phenomenon of bone resorption. RANKL a membrane bound or soluble protein belonging to is primarily produced in osteoblastic lineages & activated T-cells, stimulates osteoclasts differentiation & activation & inhibits osteoclasts apoptosis. Binding of RANKL to RANK expressed on the surface of osteoclasts & their precursors, promotes osteoclasts differentiation & activation [3–7].
In periodontitis, infiltrated leucocytes produce inflammatory mediators such as interleukin 1 & prostaglandins E2 which affect the RANKL & OPG expression by osteoblasts, periodontal fibroblast & gingival fibroblasts [6].
Bone resorption is regulated by the interplay of Receptor Activator of NF-kB Ligand (RANKL) and osteoprotegerin (OPG), two molecules belonging to the tumour necrosis factor ligand and receptor families, respectively [8]. RANKL, produced by various cells, stimulates RANK receptor on pre-osteoclasts and subsequently their differentiation into multi-nucleated osteoclasts, which will resorb bone. On the contrary, OPG inhibits the action of RANKL by binding to it, thus preventing osteoclast differentiation and bone resorption. A local increase of the RANKL/OPG ratio is observed at sites exhibiting enhanced bone resorption [7].
Thus, if the molecular mechanism of alveolar bone were elucidated in detail, prognosis of the periodontal lesion will be more accurately diagnosed & appropriate treatment modalities will be proposed. Since the RANKL-OPG system is crucial for controlling bone resorption, imbalances in its expression may cause a switch from a physiological to a pathological bone loss in different sites.
The study aimed to identify & compare RANKL positive cells in the gingival tissues of the clinically healthy control & chronic periodontal disease patients and evaluate for immunohistochemical evidence of RANKL protein in gingival tissue samples.
Materials and Methods
Patients were randomly selected from Sree Balaji Dental College for this study, 30 subject each for were grouped as (test & control). Controls are assigned as Healthy Gingival & Test as Chronic Periodontitis Patients. All of the participants were informed in detail of the procedure & signed an informed consent form in advance of their participation in this study. The study protocol was approved by the institution’s ethical committee. Prior to the examination a thorough medical and dental history was taken.
Inclusion Criteria: Included either sex (Males & Females), age 25-55y, at least 14 teeth should be present, ≥ 8 teeth that suffered radiographic bone loss, at least 3 teeth with PPD (periodontal probing depth) ≥ 6mm, Systemically healthy individuals, Non-smokers.
Exclusion Criteria: Patient with systemic diseases, patients with medical disorders such as diabetes mellitus, immunological disorders, hepatitis, history of alcoholism and smoking; liver, kidney or salivary gland dysfunction; infectious diseases; inflammatory bowel disease; rheumatoid arthritis; granulomatous diseases; diabetes; or undergoing or having undergone organ transplant or cancer therapy. In addition, use of glucocorticoids, cyclo-oxygenase inhibitors, bisphosphonates, antibiotics or immunosuppressant medication during the past 6 months; need for antibiotics for infective endocarditis prophylaxis during dental procedures; acute illness symptoms (i.e., fever, sore throat, body aches, or diarrhoea); orthodontic appliances; or presence of an oral mucosal in-flammatory condition (e.g., aphthous stomatitis, lichen planus, leukoplakia, or oral cancer, occlusal trauma and those who had antibiotic [6,9]. Pregnancy or Lactating mothers, antibiotics, anti-inflammatory & contraceptives therapy for the past 2 wk prior to the study. Periodontal therapy is performed 3 mnth before the study & smokers are excluded from this study.
Group A Healthy individuals (Control): This study group included subjects with, clinically healthy periodontium, Probing depth ≤ 3mm, No clinical attachment loss, less than 10% bleeding sites.
Group B Chronic Periodontitis (Test) [10]: This study group included, amount of microbial plaque consistent with disease severity, had a diagnosis of generalized moderate to severe chronic periodontitis based on the criteria defined by the American Academy of Periodontology. Periodontally diseased individuals need to have at least five teeth with CAL ≥3 mm and PPD ≥ 6 mm distributed among at least two quadrants, with bleeding on probing and radiographically determined bone loss [6].
Intra oral examination was done using mouth mirror and William’s periodontal probe. Periodontal evaluation was done by measuring the gingival bleeding index dichotomous (present/absent), probing pocket depth (PPD) and clinical attachment level (CAL) [11].
Sample collection: Gingival tissue was obtained from systemically healthy subjects with chronic periodontitis during conventional periodontal flap surgery.
Tissue samples of healthy group were harvested from patients, who are undergoing wisdom tooth extraction, or crown lengthening procedure for prosthodontic treatment, or canine exposure for orthodontic patients.
Clinical evidence of need (dental decay, bony or soft tissue impaction, orthodontic need) for third molar extraction as determined by medical, dental, and radiographic evaluation. 3rd molar was extracted only if there is no pericoronal infection, no antibiotic therapy prior extraction & also which is within study criteria there in no Clinical signs of infection and/or inflammation, or acute pain at any planned extraction site at the time of screening.
Immuno Histochemical Staining Procedure: Samples were fixed with 3.5% formaldehyde in 0.1 M phosphate buffer. Tissue is kept for 24h in 4oc, section of 4μ thickness were made. Chrome alum coated slides were used for the sections. Slides are kept in hot air oven for drying excess water. IHC section kept for 60 min in hot air oven. Placed in trisodium citrate buffer & replaced in D. H2O & placed in tris & primary antibody (anti-goat RANKL –sc-7627 Santacruz Biotechnology) is added - kept for 1 h & secondary antibody (vector-impress reagent kit) added, kept for 30min & washed in buffer & water for 5min & Counter stained with haematoxylin. A grid scan was used to determine the 0.1 *0.1-mm square field for cell counting, and five areas were randomly chosen in tissue area (* 200) of each specimen.
Statistical Analysis
The statistical analysis of association between patients’ demographic and clinicopathologic characteristics and the RANKL was analysed using independent t-tests for continuous variables in SPSS software, version 17.0 (SPSS, Chicago, USA). Patients’ demographic data included in the data analysis were age, gender. The immuno histochemical analysis, data were analysed using the INDEPENDENT student t-test to compare the RANKL-positive cells in gingival tissues of diseased sites and control non-diseased sites. The mean data were calculated as the percentage of positive cells relative to the total number of cells counted in each field. The association was considered as statistically significant if p < 0.05.
Results
RANKL cells were detected in both test & control. Differences existed in the intensities of RANKL-positive cells between diseased sites and control, healthy sites. In diseased tissues, 26 shows positivity for epithelium & connective tissue, four tissue sample shows positivity for connective tissue only.
RANKL positive cells show intense cytoplasmic staining, which appears blue in colour it is widely seen in the epithelium & connective tissue of the gingiva.
Results showed RANKL-positive cells were widely distributed in the gingival tissue of patients with chronic periodontitis [mean ± standard deviation (SD), 61.76% ± 10.49%]. Percentage of positive cells for healthy sites ranges between [9.92% - 19.33%] & for diseased site ranges between (38.21% -79.51%) [Table/Fig-1]
Independent T-test statistics for calculation of receptor activator of nuclear factor-kappa B ligand (RANKL)-percentage of positive cells (%).,
| Percentage of Positive cells | n | Minimum | Maximum | Mean | Standard deviation (SD) |
|---|
| Healthy | 30 | 9.92 | 19.38 | 16.49 | 2.56 |
| Chronic periodontitis | 30 | 34.36 | 79.51 | 61.76* | 10.64 |
*Asymmetrically significant (two-tailed), p < 0.05
The INDEPENTDENT t-test showed a significant difference in the RANKL-positive cell percentage between diseased sites of patients with chronic periodontitis and those of healthy individuals (P < .05).
Total amount of positive cells present in diseased site per sample ranges between (687.21 – 1442.09) cells, whereas for healthy site it ranges between (198.45 – 383.54) cells. The total amount of cells present ranges between (mean ± standard deviation (SD), 1235.36 ± 212.97 cells) is seen in [Table/Fig-2]. The INDEPENTDENT T-test showed a significant difference in the total no. of RANKL-positive cell between diseased sites of patients with chronic periodontitis and those of healthy individuals (p < .05).
Independent T-test statistics for calculation of receptor activator of nuclear factor-kappa B ligand (RANKL) - Total number of cells.,
| Percentage of Positive cells | n | Minimum | Maximum | Mean | Standard deviation (SD) |
|---|
| Healthy | 30 | 198.5 | 387.80 | 330.00 | 51.39 |
| Chronic periodontitis | 30 | 687.20 | 1590.30 | 1235.36* | 212.97 |
*Asymmetrically significant (two-tailed), p < 0.05
Bar diagram depicting the content, distribution of RANKL cells in either sex are illustrated in [Table/Fig-3]. RANKL cells are equally (50%) distributed in either sex in healthy individuals, whereas in diseased individuals it is 46.7% in females & 53.30% in males [Table/Fig-4]. In diseased subjects, males have RANKL positive cells marginally increased in percentage compared to females. There is no significant difference between either sex in healthy individuals.
Total number of the receptor activator of nuclear factor-kappa B ligand (RANKL) positive cells in gingival tissue obtained from either sex

Independent T-test statistics for calculation of receptor activator of nuclear factor-kappa B ligand (RANKL) - Total number of cells percentage in either sex
| RANKL positive cells in either sexes | n | Male | Female |
|---|
| Healthy | 30 | 50% | 50% |
| Chronic periodontitis | 30 | 53.30% | 46.7% |
Results show there is no significant difference in age group either in males or females & in healthy or diseased individuals [Table/Fig-5]. There is no significant difference in presence of RANKL positive cells in different age group in healthy & diseased individuals.
Independent T-test statistics for calculation of receptor activator of nuclear factor-kappa B ligand (RANKL) - Total number of cells distributed in age group
| RANKL positive cells in different age group | n | Mean | Standard deviation (SD) |
|---|
| Healthy | 30 | 30.83 | 3.931 |
| Chronic periodontitis | 30 | 41.83 | 6.33 |
Results show there is no significant correlation in gingival bleeding index & RANKL positive cells. There is statistically significant correlation in severity of periodontal status, PPD, CAL in test group individuals [Table/Fig-6] presence of RANKL positive cells in different age group in diseased individuals. There are no significant correlation healthy sites for PPD, CAL & gingival bleeding site.
Spearman’s Correlations: Total number of RANKL positive cells, percentage of RANKL positive cells correlated with PPD, CAL.,
| Test Group Periodontal probing Depth and Clinical Attachment Los | Total number of Rankl Cells | % of Rankl positive cells |
|---|
| Test Group Periodontal probing Depth and Clinical Attachment Loss | Correlation Coefficient | 1.000 | .620** | .620** |
| p Value | | .000 | .000 |
| N | 30 | 30 | 30 |
| Total number of Rankl Cells | Correlation Coefficient | .620** | 1.000 | 1.000** |
| p Value | .000 | | |
| N | 30 | 30 | 30 |
| % of Ranks Positive Cells | Correlation Coefficient | .620** | 1.000** | 1.000 |
| p Value | .000 | | |
| N | 30 | 30 | 30 |
**. Correlation is significant at the 0.01 level (2-tailed)
Discussion
Periodontitis is a complex, multifactorial process affected by bacterial plaque-components and host defense mechanisms. Inflammation of the periodontitium may lead the destruction of the underlying ligament and alveolar bone. Receptor activator of NF-KB ligand (RANKL), a novel TNF receptor-related protein is an important factor for osteoclast differentiation and activation. Osteolysis by osteoclast has been demonstrated in periodontitis, Armitage G, Holt SC et al., [2]. Animal experiments using a chimeric model of Periodontitis support the view that Rankl is the key molecule regulating bone destruction in periodontitis [6,9], However, Fu-Hsiung Chuang et al., reported increased expression of RANKL, RANK and OPG in normal human oral mucosa. Strong cytoplasmic staining of RANKL has been noted in the basal layers. This also correlates our findings that RANKL positive cells are present in both healthy and diseased tissue [12].
RANK, RANKL cells have also been found in dental tissues & cells in human deciduous teeth [13] & study done by Haynes DR et al., [14,15] reported that OPG and RANKL have been detected by immunohistochemistry on odontoblasts, ameloblasts and pulp cells in mice which is also in accordance with our study that RANKL positive cells are presents in healthy gingival tissue samples.
Out of 30 diseased sites analysed 26 showed positivity in epithelium and connective tissue, four show only connective tissue positivity this is correlated with the study done by Fu-Hsiung Chuang et al., [12] that in basal layer of the epithelium in normal buccal mucosa RANKL cells are present.
In diseased tissues RANKL positive cells are significantly increased compared to healthy tissues [Table/Fig-7,8]. Our study demonstrated that total amount of RANKL positive cells is significantly (p<0.05), increased in chronic periodontitis compared to healthy tissues which correlates with the study done by Rolando Vernal et al., [14,15] reported that total amount of RANKL is significantly higher in active versus in active sites (p<0.05). In periodontal disease, increased concentrations of RANKL are found in diseased tissues, and upset balance with concentrations is associated with disease severity. This is also in accordance with the study of Crotti et al., [9], who concluded that there is an elevation of RANKL in tissue adjacent to alveolar bone loss & also another study done, using RT-PCR analysis of gingival tissues demonstrated that RANKL positive cells were increased in periodontitis patients compared to healthy individuals [14].
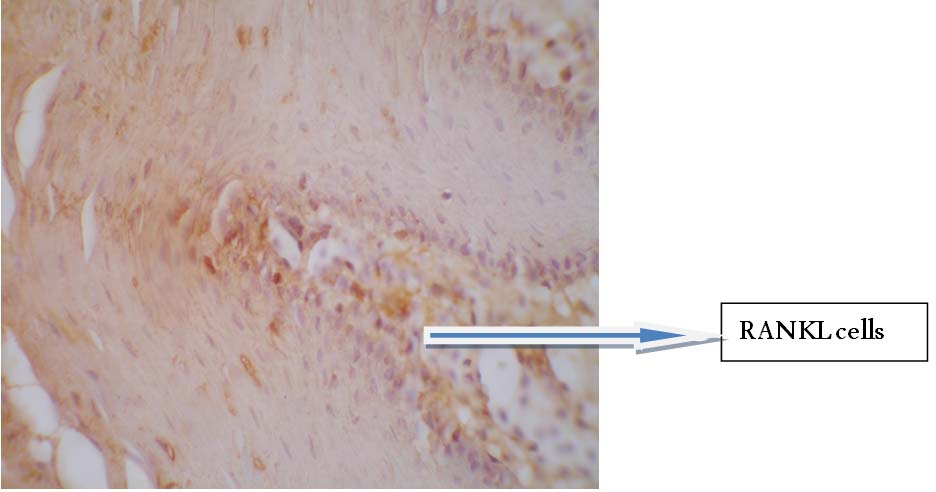
Strong cytoplasmic staining of RANKL positive cells in diseased tissues. (40X microscopic Image)
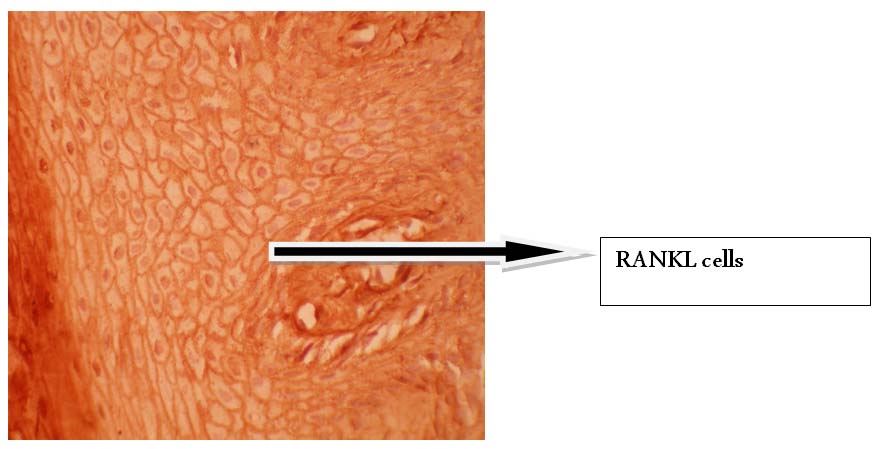
Shows RANKL positive cells in healthy tissue. (40X microscopic Image)
The infiltration of chronic inflammatory cells into the gingival tissues is likely to be a key step in the inflammatory process leading to alveolar bone lysis in periodontitis. Many of these inflammatory cells, such as blood derived monocytes, have the potential to become osteoclasts [6].
Although, periodontitis is one of the most prevalent human pathologies associated with bone loss, the mechanism of osteoclast formation and bone resorption in this disease is poorly understood. While it is widely accepted that inflammatory cytokines known to stimulate bone resorption, such as IL-1b, TNF, PGE2, c-interferon and IL-6, are present in human periodontitis tissues [8,16] the role of the key mediators of osteoclast activity, RANK, RANKL and OPG are yet to be determined [16].
While many studies have also shown that RANKL and OPG are expressed by the types of cells present in the gingival tissues, human periodontal ligament cells has been shown to express both RANKL and OPG mRNA in vitro [17].
The RANKL produced by osteoblasts can act in either a paracrine manner to activate osteoclast activity directly, or in an autocrine manner, similar to that of IL-6, to stimulate osteoblasts to produce further RANKL, which directly activates osteoclasts. Numerous cytokines, such as IL-1a, IL-1b, TNF-a, IL-6 and IL-8, are known to modulate the OPG/ RANKL/RANK system and increase bone resorption [12].
Thus alveolar bone destruction observed in Chronic Periodontitis is at least partially to the action of osteoclasts & which is mediated by RANKL.
Conclusion
The study demonstrated that, presence of RANKL positive cells in both in healthy & chronic periodontitis gingival tissues. In diseased tissues RANKL positive cells are significantly increased compared to healthy tissues. The study finding can be useful in planning new treatment approach for periodontal disease management. Further studies with a larger sample size, different age & sex groups with varying clinical parameters are needed to elucidate in detail the RANKL mechanism and its role as a key mediator of osteoclast activity in chronic periodontitis.
*Asymmetrically significant (two-tailed), p < 0.05
*Asymmetrically significant (two-tailed), p < 0.05
**. Correlation is significant at the 0.01 level (2-tailed)