Case Report
A 12-year-old boy, presented with low-grade fever and altered sensorium, moving all the four limbs initially, but gradually developed decerebrate posture with shallow buried breathing.
On General examination: Glassgow coma scale (GCS) was E2M5Vt. Left pupil showed sluggish response. On examination of fundus, blurred optic disc with obliteration of physiological cup was seen. All brain stem reflexes were intact, patient could move all the limbs equally. Pulse, blood pressure and tone of both upper & lower limbs were within normal limits.
On Systemic examination: Patient had shallow buried breathing for which he was intubated. No organomegaly was seen. Cardiovascular, Gastrointestinal & Genitourinary systems showed no obvious abnormality.
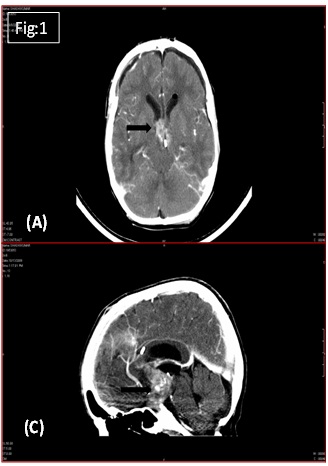
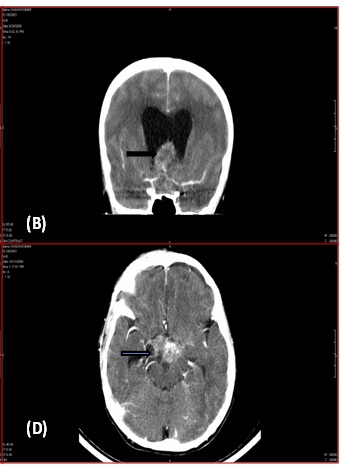
Preoperative CT Scan showed extra-axial enhancing lesion (3.3X2.5X3.7cms) [Table/Fig-1a,b] in sellar region causing obstruction of suprasellar (intra-peduncular cistern) along with a hyper dense area in periphery of lesion with dilatation of frontal/ temporal/ occipital horns of lateral ventricle. Fourth ventricle appeared normal [Table/Fig-1c,d].
Pre- operative CT Scan showing extra-axial enhancing lesion.
CT Scan showing recurrent lesion (after 3 months of complete surgical clearance)
On Biochemical investigation; β-CEA: 1.2 ng/mL (Normal: <2.5 ng/mL), β-HCG: 340.4 mIU/mL (Normal: <5mIU/mL), αFP: 29.3 ng/mL (Normal: <10ng/mL) were found. Hence, it is seen that levels of tumour markers, i.e. β-HCG and αFP are significantly elevated, which aided in the diagnosis further.
Micro-surgical trans-callosal interferential approach was undertaken and total excision of tumour was done under general anaesthesia.
Grossly, multiple nodular grey-white to grey-brown tissue bits were received, together weighed around 6g, largest bit measured 2x1.5x1 cms. On cut section, grey-white and hemorrhagic areas were identified.
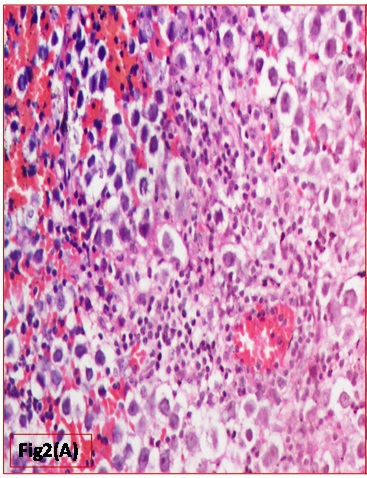
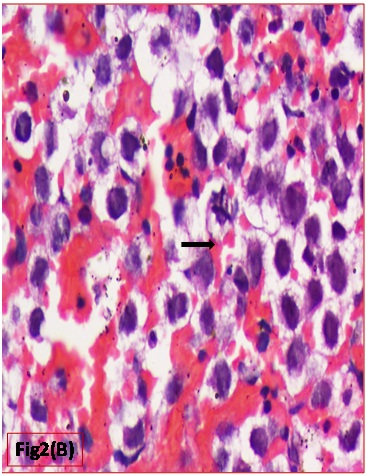
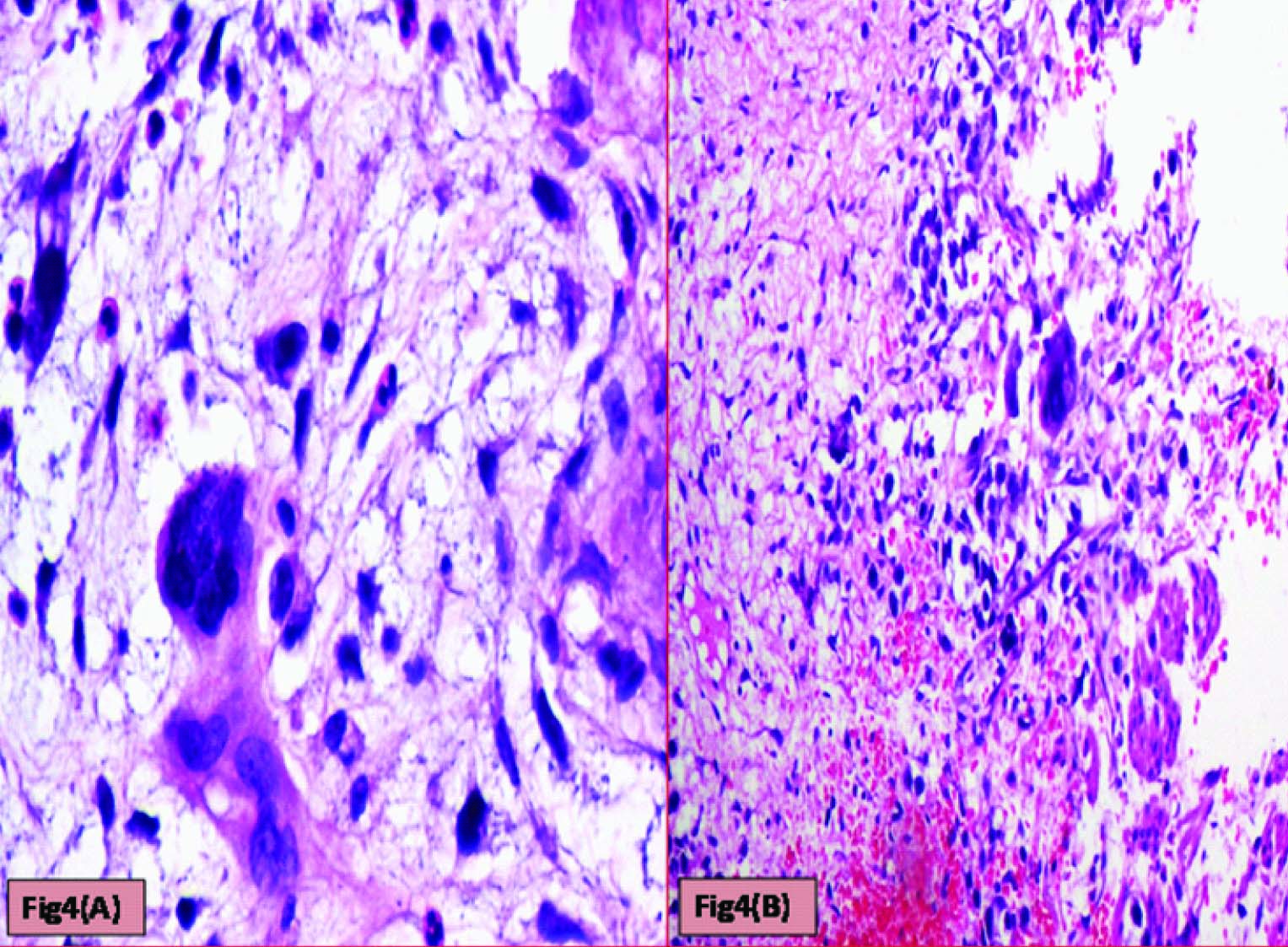
Histopathological evaluation showed a tumour with monomorphic large germ cells with enlarged centrally located vesicular nucleus, prominent nucleolus and moderate clear cytoplasm arranged in sheets, divided into lobules by fibrous septa with lymphocytic infiltrates and stromal hemorrhage; foam cells were also noted [Table/Fig-2a,b]. Also seen were immature glands lined by columnar cells with hyperchromatic nuclei and subnuclear- vacuolation surrounded by atypical spindle shaped stromal cells and foci of immature cartilage [Table/Fig-3]. Large areas of sinusoidal vascular channels surrounded by cytotrophoblasts and syncytiotrophoblasts were seen suggestive of choriocarcinoma [Table/Fig-4a,b].
Monomorphic large germ cells representing Germinoma (H&E: 200X)
Atypical mitotic figure seen (H&E: 400X)
Immature gland lined by columnar cells with hyperchromatic nuclei representing Immature Teratoma (H&E: 200X)

Cytotrophoblasts & Syncytiotrophoblasts with (a) Myxoid areas (H&E: 400X) (b) Hemorrhagic areas (H&E: 200X)
Based on the above findings a final diagnosis of Mixed Malignant Germ Cell Tumour (Immature Teratoma + Germinoma + Choriocarcinoma) was made.
Three months later, the child presented with nocturnal urinary incontinence and difficulty in walking. CT scan [Table/Fig-1c&d] showed a lesion in suprasellar region with evidence of hydrocephalus. Histopathological examination of excised lesion showed tumour recurrence. Patient was advised Radiotherapy and further follow up.
Discussion
Pediatric Germ Cell Tumors involving CNS are uncommon. Primary involvement of the CNS occurs in around 18% cases, with metastatic involvement being more common [1]. Just like their extra-cranial counterparts, they present a wide array of diseases [2,3]. They may be seen from birth and throughout childhood with varying incidence depending upon patient’s age and gender. They arise primarily in two locations in the center of brain, the suprasellar in 40% cases and pineal region in 50% cases. 5-10% cases have both suprasellar and pineal gland tumour called as doublet lesions [2–4].
These tumours are considered to arise from nests of embryonic cells (rests) located in the midline, both intra-cranially as well as extra-cranially[5]. Malignant GCTs are classified in similar way as their gonadal counterparts, i.e. germinoma, (being the most common), followed by teratoma and a number of Non-germinomatous tumours including embryonal Carcinoma, Choriocarcinoma, endodermal sinus tumours & combination of these tumours have been reported.
Both, suprasellar and pineal region tumours vary in their clinical presentation. Suprasellar tumours present with classic triad of visual disturbances, diabetes insipidus and hypopituitarism in about 20% cases. Pineal region tumours show signs of increased intracranial pressure, ophthalmologic alterations, ataxia, seizures, behavioural abnormalities.
Malignant GCTs represent approximately 3% neoplasm in children, peak incidence at 10-21 years. There is an overall male preponderance (as in the present case) [2]. Pure Germinomas (non-secreting tumours) are much more responsive to radiation and chemotherapy than Non-germinomatous Germ cell tumours (secretory tumours). Hence, it becomes very important in correctly diagnosing the different components in the mixed variant of intracranial GCT, as it significantly affects the treatment modalities.
Various hypothesis have been postulated regarding formation of extra-gonadal GCTs. First being the Telium’s Germ cell theory which states that sometimes primordial germ cells migrate aberrantly during embryogenesis, undergo malignant transformation & ultimately giving rise to extra-gonadal GCTs. Secondly, some believe in the Embryonic cell theory which states that GCTs result because of mismigration of pleuripotent embryonic cells.
The World Health Organization has classified CNS GCTs into the following major groups [6].
Choriocarcinoma.
Embryonal carcinoma.
Germinoma.
Mixed germ cell tumours.
Teratoma.
Immature.
Mature.
Teratoma with malignant transformation.
Yolk sac tumours.
Germinomas in brain has histological similarities with seminoma in testes & dysgerminoma in ovary with very uniform looking large round cells with vesicular nuclei, clear/finely granular eosinophilic cytoplasm, resembling primordial germ cells. Stroma, typically is seen with high lymphocytic infiltration.
Hence, it is very important to identify all components in malignant GCTs and rule out any kind of metastasis from gonads by proper clinical history, general and systemic examination. Histology will suffice the diagnosis when the specimen is sufficient for evaluation & well preserved without artifact. But when specimen is either very small or there is too much lymphocytic infiltration, IHC plays a major role. GCTs show diffuse cytoplasmic positivity with PLAP and C-kit (CD117). Currently, OCT4 (POU5F1, OCT3, OTF3) a highly specific and sensitive marker for primary intracranial germinoma has been recognised [7].
Foci of Choriocarcinoma in a mixed GCT must be differentiated from seminoma with syncytiotrophoblasts and other sex-cord stromal tumours. Intracranial choricarcinomas are rare, constituting 5% of pineal masses and 10% of all intracranial GCTs. Apart from their characteristic histological features (with syncytiotrophoblast & cytotrophoblast, mitosis, extensive hemorrhage and necrosis), they typically show increased CSF & plasma β- HCG, which are helpful in establishing the diagnosis. In the present case, biochemical serum markers aided in the diagnosis. Useful IHC markers include β- HCG, pregnancy specific beta-1 glycoprotein (SP1) and inhibin.
IHC also plays an important role in differentiating choriocarcinoma from other GCTs with syncytiotrophoblastic cells. OCT 3/4 will be negative in syncytiotrophoblastic components of choriocarcinoma but positive in those of seminoma or embryonal carcinoma [7].
The third component Observed in the present case was immature teratoma. IHC marker SOX-2 highlights primitive, neuroepithelial tissue in immature teratoma whereas negative in mature teratoma. Further, epithelial component will be positive for keratin & neuronal component stain positive for S-100 or GFAP [8].
In addition to the microscopic appearance of the various CNS GCTs, tumour markers (proteins secreted by the tumour cells) found in the serum and cerebrospinal fluid (CSF) aid in diagnosis [Table/Fig-5,6] [9].
According to tumor marker
| Tumor Type | Beta-HCG | AFP | PLAP | c-KIT |
|---|
| Choriocarcinoma | + | - | +/- | - |
| Embryonal carcinoma | - | - | + | - |
| Germinoma (syncytiotrophoblastic) | + | - | +/- | + |
| Immature teratoma | +/- | +/- | - | +/- |
| Mature teratoma | - | - | - | - |
| Mixed germ cell tumor | +/- | +/- | +/- | +/- |
| Pure germinoma | - | - | +/- | + |
| Yolk sac tumor | - | + | +/- | - |
AFP = alpha-fetoprotein; HCG = human chorionic gonadotropin; PLAP = placental alkaline phosphatase
Serum and Cerebrospinal Fluid Markers
| Tumor Type | Beta-HCG | AFP |
|---|
| Choriocarcinoma | +++ | - |
| Embryonal carcinoma | - | - |
| Germinoma | (+/-) | - |
| Teratoma | - | (+) |
| Yolk sac tumor | - | +++ |
AFP = alpha-fetoprotein; HCG = human chorionic gonadotropin; + = positive; - = negative; ± = equivocal; +++ = strongly positive; (±) equivocal, not diagnostic; (+) = positive, not diagnostic.
To summarize, different immunohistochemical markers which can aid in differential diagnosis of various germ cell tumours are mentioned in [Table/Fig-7].
Positive and negative immunohistochemical markers for germ cell tumors
| Tumor types | Positive markers | Negative markers | Additional positive markers |
|---|
| Seminoma | OCT4, NANOG, CD117 | Sox2, glypican-3, CD30 | D2-40 |
| Embryonal cell carcinoma | OCT4, Sox2, NANOG, CD30 | CD117, glypican-3 | Pancytokeratin |
| Yolk sac tumor | Glypican-3, pancytokeratin | OCT4, NANOG, Sox2, CD30, CD117 | AFP HepPar-1 |
| Immature teratoma | Sox2, pancytokeratin | OCT4, NANOG, CD117, CD30, glypican-3 | GFAP |
| Choriocarcinoma | Glypican-3, pancytokeratin | OCT4, NANOG, CD30, Sox2, CD117 | HCG, HLA-G |
A recent study of intracranial GCTs revealed expression of germ cell–specific proteins comprising MAGE-A4, NY-ESO-1 & TSPY, which are associated with embryonic stem cell pluripotency. This indicates that GCTs may originate from primordial germ cells [10,11].
Increased copies of X chromosome are seen in CNS GCTs; most frequent genotype abnormality is XXY, similar to that in Klinefelter syndrome. Individuals with Klinefelter syndrome, Down syndrome & with neurofibromatosis type 1 are prone to develop intracranial GCTs [10,11].
Recently introduced SALL4, a novel diagnostic marker for primary GCT of CNS, with 100% sensitivity for germinomas, embryonal carcinomas & yolk sac tumours of CNS can be used to confirm a primary CNS tumour to be a germ cell tumour [12].
As these tumours have a tendency to spread throughout the Central Nervous System, complete CNS disease evaluation, including brain and spine MRI, measurement of CSF and serum-tumour markers and CSF cytology are essential. Treatment of these rare GCTs of brain is associated with high cure rates. Adequate staging and histology of CNS GCTs are important factors used to stratify patient into appropriate treatment group.
Over the last two decades, advances in diagnostic imaging, surgery, radiotherapy and chemotherapy have led to drastic improvements in prognosis of malignant GCTs especially pure germinoma. But the rarity and complexity of mixed type of GCTs, demand that appropriate diagnosis and treatment be undertaken.
Conclusion
Treating different Germ Cell Tumour components within the same tumour, each with different biological behaviour, demand different approaches to treatment. It is essential that an accurate diagnosis is made to ensure that a patient is neither undertreated nor overtreated.
Genomic analysis of GCTs has shown distinct mRNA and miRNA profiles, which when correlated with histological differentiation and clinical outcome in future, May serve as novel therapeutic targets.
New drug programs must be developed in order to improve the cure rate for children and prevent recurrences.
AFP = alpha-fetoprotein; HCG = human chorionic gonadotropin; PLAP = placental alkaline phosphataseAFP = alpha-fetoprotein; HCG = human chorionic gonadotropin; + = positive; - = negative; ± = equivocal; +++ = strongly positive; (±) equivocal, not diagnostic; (+) = positive, not diagnostic.
[1]. Göbel U, Schneider DT, Calaminus G, Haas RJ, Schmidt P, Harms D, Germ-cell tumours in childhood and adolescenceAnn Oncol 2000 11(3):263-71. [Google Scholar]
[2]. Jennings MT, Gelman R, Hochberg F, Intracranial germ cell tumours, Natural history and pathogenesisJ Neurosurg 1985 63:166-67. [Google Scholar]
[3]. Lin IJ, Shu SG, Chu HY, Primary intracranial germ cell tumour in childrenJ Neurosurg 1998 88:3576-80. [Google Scholar]
[4]. Rutka JT, Hoffman HJ, Drake JM, Suprasellar and sellar tumours in childhood and adolescenceNeurosurg Clin N Am 1992 3:803-20. [Google Scholar]
[5]. Nomura K, Epidemiology of germ cell tumours in Asia of pineal region tumourJ Neuro Oncol 2001 54:211-17. [Google Scholar]
[6]. Miyanohara O, Takeshima H, Kaji M, Diagnostic significance of soluble c-kit in the cerebrospinal fluid of patients with germ cell tumoursJ Neurosurg 2002 97:177-83. [Google Scholar]
[7]. Sato K, Takeuchi H, Kubota T, Pathology of intracranial germ cell tumoursProg Neurol Surg 2009 23:59-75. [Google Scholar]
[8]. Palmer RD, Foster NA, Vowler SL, Roberts I, Thornton CM, Hale JP, Malignant germ cell tumours of childhood: new associations of genomic imbalanceBr J Cancer 2007 96(4):667-76. [Google Scholar]
[9]. Rosenblum MK, Matsutani M, Van Meir EG, CNS germ cell tumours. In: Kleihues P, Cavenee WK, editorsPathology and Genetics of Tumours of the Nervous System 2000 Lyon, FranceInternational Agency for Research on Cancer:208-14. [Google Scholar]
[10]. Schneider DT, Zahn S, Sievers S, Molecular genetic analysis of central nervous system germ cell tumours with comparative genomic hybridizationMod Pathol 2006 19(6):864-73. [Google Scholar]
[11]. Wang HW, Wu YH, Hsieh JY, Peaadiatric primary central nervous system germ cell tumours of different prognosis groups show characteristic miRNome traits and chromosome copy number variationsBMC Genomics 2010 11:132 [Google Scholar]
[12]. Kaiyong Mei, Diagnostic utility of SALL4 in primary germ cell tumours of the central nervous system: a study of 77 casesModern Pathology 2009 22:1628-36. [Google Scholar]