Correction:
This article has been updated on 1 Aug 2016, to see correction please see link which is given below:
http://jcdr.net/article_fulltext.asp?issn=0973-709x&year=2016&volume=10&issue=7&page=ZZ01&issn=0973-709x&id=8393
Background: Hepatotoxicity is one of the inevitable side effects of long term use of medicines in many different disorders, chronic use of alcohol and in certain infectious disorders. Even though there are few effective drugs to treat such hepatotoxicity, mortality due to hepatotoxicity is increasing day by day. Therefore, in search of alternative and more effective medicine we found that Caesalpinia bonduc(CB), a shrub grows in hotter places of south Asia has been effectively used to treat such hepatotoxicity in folk medicine.
Aim: Aim of the study is to scientifically evaluate the hepatoprotective nature of aqueous extract of CB using Carbon tetrachloride(CCl4) induced hepatotoxic rat model.
Materials and Methods and Result: Elevated levels of blood ALT, AST and ALT enzymes were found in CCl4 induced hepatotoxic rat models. Treating these animals with CB either prior or after the induction of hepatotoxicity, shows significant decrease in the levels of ALP, AST, and ALT in their blood in comparison with the untreated hepatotoxic group. Additionally, histologically, hepatotoxic rats show necrotic changes & vacuolation in their hepatocytes, altered hepatic architecture and congested hepatic sinusoids. However, such histopathological adverse changes were minimized when these animals treated with CB.
Conclusion: Results of the present study indicate that CB acts as both preventive and curative hepatoprotector
Introduction
Drug-induced haepatoxicity is a major health issue worldwide, and managing this problem is not only a challenge for health care professionals but also the pharmaceutical industry and drug regulatory agencies.In addition, old age, poor nutritional status, infection with hepatitis virus, HIV and excess alcohol consumption are considered as other predisposing factors for the hepatotoxicity. In these conditions, the effective hepatoprotecitve drug is essential to overcome the hepatotoxicity and its consequences. Even though there are few such hepatoprotective drugs are available, the alternative medicine such as herbal remedies are getting more popular worldwide because of their efficacy, minimal side effects and cost efficiency and are prescribed widely, even when their biological active compounds are unknown [1]. Therefore, studies with plant extracts are essential to assess their effectiveness, mode of action and its safety. Many herbal preparations have been in use as successful and safe alternative treatment for hepatotoxicity. One such plant is Caesalpinia bonduc (CB), is reputed to possess multiple therapeutic properties. The leaves of this plant are traditionally used for the treatment of tumor, inflammation and liver disorders [2]. It has been also known for other therapeutic properties like: antipyretic, antidiuretic, anthelmintic and antibacterial, [3] Anticonvulsant, [4] Anti-anaphylactic, antidiarrheal and antiviral [5], Antiasthmatic, [6] Anti-inflammatory, [7] Antiamebic and antiestrogenic, [8] Abortificant [9]. As the CB has been also used to treat various kinds of hepatotoxicity cases in folk medicine, this study is aimed to scientifically evaluate the hepatoprotective effect of CB using a hepatotoxic rat model.
Materials and Methods
Chemicals and plant materials: CCl4 was procured from Merck Ltd., Mumbai, other chemicals used in this study were procured from local reliable vendors. Leafs of CB were collected in month of February from the botanical garden of SDM College of Ayurveda, Udupi and were authenticated by the “Dhravyaguna” expert in SDM college of Ayurveda, Udupi.
Preparation of aqueous extract (Hot maceration method): Leaves of CB were shade dried and powered. Leaf powder (200g) was then dissolved in 1500ml of distilled water and decoction was prepared at 75-800C. Decoction was then cooled and filtered. Finally the filtrate was evaporated to dryness using lyophilizer.
Preparation of the test material: Carboxy Methyl Cellulose (CMC) stock solution is prepared by dissolving 250 mg of CMC in 100 ml of PBS(Phosphate Buffer Solution). Dilution of aqueous extract of CB is prepared by dissolving 500 mg of aqueous extract it in the 10ml CMC of 0.25% w/v.
Animals: After obtaining the required clearance from the institutional animal ethical committee, healthy female Albino rats(150- 200gms) were maintained on standard environmental condition at room temperature of 270C with 12hr day/night cycle incentral animal house as per Committee for Control and Supervision on Experiment on Animals guidelines (CPCSEA No. 94/999), Manipal University, Manipal. They were fed with a standard pellet diet and water ad libitum. Animal studies were approved by the institutional animal ethics committee (IAEC/KMC/34/2009-2010).
Toxicity study: The toxicity test of aqueous extract of CB was done as per stair case methods [10]. Ten Rats were divided into groups of six animals each. The control group received saline and the other groups received single oral dose of 100, 500, 1000, 2000, 3000, and 4000 mg/kg body weight of CB extract respectively. Then, animals were observed for various toxic signs and symptoms continuously for the first 4hr and further observed for 7 days. Based on observed results, the dose of 500mg/kg body weight was considered as optimal and safe dose for further experiments.
Treatment protocol: Animals were divided in to five groups of six animals each. Aqueous extract of CB was administered orally by using intra-gastric tube at the dose of 500mg/kg body weight.
0.5 mg/kg body weight of CCl4 was injected intraperitoneally to induce hepatotoxicity [11].
Study was conducted using following groups-
GROUP I : Control
GROUP II: Tox5 (CCl4 0.5mg/kg, daily once for 5 Days)
GROUP III: Tox10 (CCl4 0.5mg/kg, daily once for 10 Days)
GROUP VI: Preventive (CB extract once daily for 5days along with CCl4)
GROUP V: Curative (CCl4 for 10 days followed by CB extract for last 5days)
Biochemical estimation: At the end of the treatment, blood was collected by retro orbital artery bleeding and the samples were kept in the room temperature for 1 hour. Further, the samples were centrifuged for 5 minutes at 1000 rpm to separate the serum. Thus the serum procured is used to estimate ALT (Alanine aminotransferase), AST (Aspartate aminotransferase) and ALP (Alkaline phosphatase) levels by using standard liver function kits as per the instructions from the manufacturer(Aspen laboratories, India).
Histopathological studies: Once the animals were sacrificed by cervical dislocation the liver was excised, washed and quickly fixed in 10% formalin, dehydrated using graded alcohol (50%, 70%, 90% and 100%), cleared in xylene and embedded in paraffin wax. Serial sections of 5μ thick section were taken by using rotatory microtome. The deparaffinized sections were stained with haematoxylin and eosin. Sections were then observed under microscope for the possiblehistopathological changes in the liver architecture and photographed and analyzed with the help of an pathologist.
Statistical Analysis
The data were analysed using Prism 5.03 Demo Version (Graph Pad Software Inc., La Jolla, CA, USA) by one-way analysis of variance (ANOVA), comparison of the mean values of various groups was performed by Bonferroni’s Multiple Comparison Test. p< 0.01 was considered significant.
Results
The biochemical analysis shows that, CCl4 administration for 5 and 10 days (Group II & Group III respectively) exhibits significant dose dependent elevation in the serum levels of ALT, AST, ALP indicating acute hepatocellular damage and biliary obstruction in comparison to control Group I. However, administration of aqueous extract of C Balong with CCl4 showed a significant decrease in these enzyme levels, almost closer to that of control group. Further, administration of CB along with the last 5 days of CCl4 treatment also reduced the levels of ALT, AST and ALP when compared to the animals treated with CCl4 alone for 10 days [Table/Fig-1].
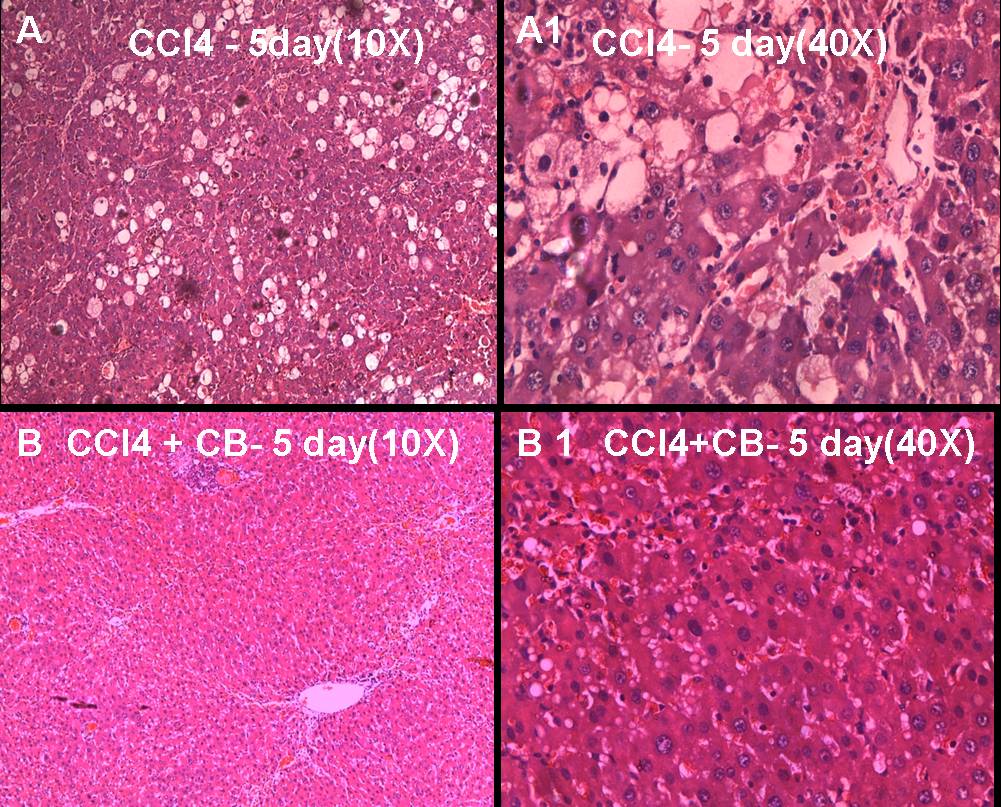
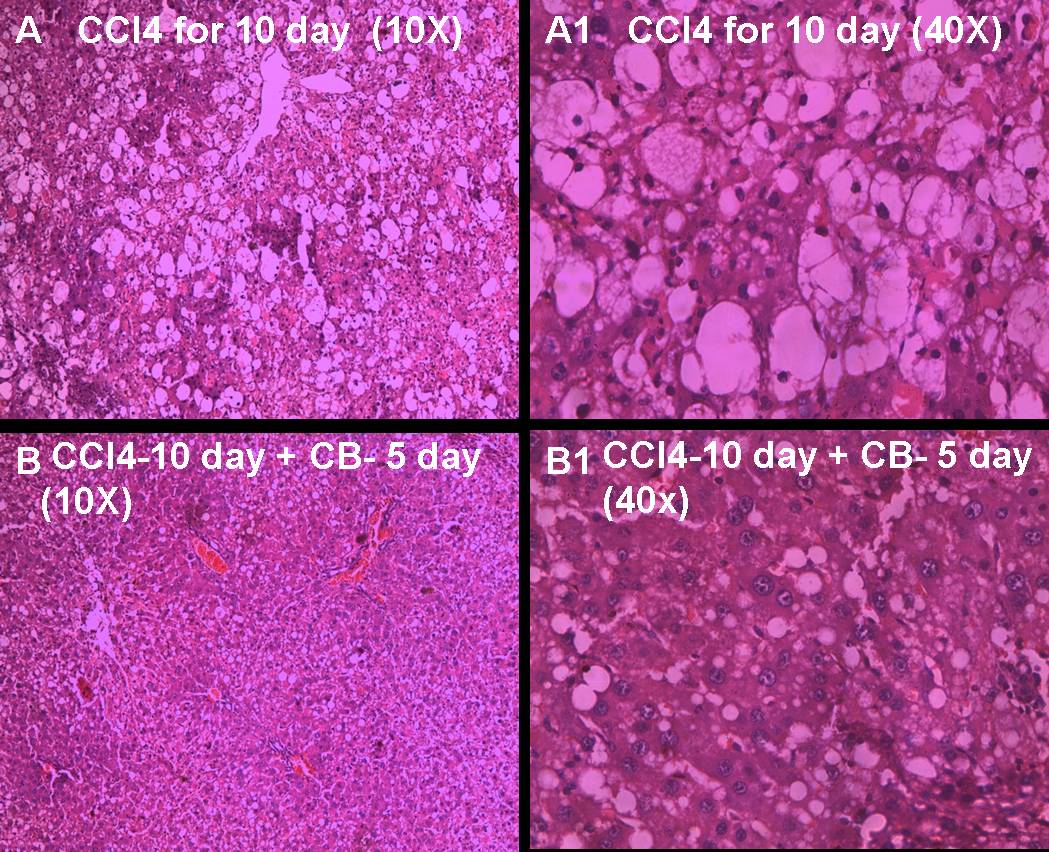
Results of histopathological studies [Table/Fig-2A-D,Table/Fig-3A-D] provided supportive evidence for biochemical analysis. The animals treated with CCl4 showed loss of normal architecture of the liver with derangement and disfigurement of hepatocytes with micro and macro vacuoles, degenerating nuclei, congestion of sinusoids and central veins. These changes were milder in animals treated with CCl4 for five days [Table/Fig-3A,B] and severe in animals treated with CCl4 for ten days [Table/Fig-2C,D]. When animals treated with aqueous extract of CB along with CCl4 (Preventive group) for five days, the normal architecture of the liver was restored with mild sinusoidal congestion and micro vacuoles in the hepatocytes and well-defined central vein when compared with animals treated with CCl4 alone for five days [Table/Fig-3a,b]. Similar findings were also found in animals treated with CCl4 for ten days along with CB for last five days. The sinusoidal congestion and vacuoles were much lesser in CB treated group in comparison with that of ten days of CCl4 treatment alone [Table/Fig-3c,d].Thus, the biochemical and histopathological results are suggestive of the hepatoprotective activity of aqueous extract of CB.
Discussion
CCl4 is universally used to induce hepatotoxicity in model for screening the hepatoprotective drugs [12] and a single exposure can lead to an increase in the level of several liver enzymes, severe centrizonal necrosis and steatosis [13]. CCl4 produces free radicals which binds to lipoprotein and leads to peroxidation of lipids of the endoplasmic reticulum [14] leading to defective plasma membrane and release of cytosolic liver enzymes into circulation. Estimation of such serum enzymes is a useful quantitative marker of the extent and type of hepatocellular damage [15]. The ideal hepatoprotective drug must possess the capacity to cure or minimize ill effects of toxicity and to restore or promote the normal functions of the liver. In the present study we have successfully induced hepatotoxicity by administration of CCl4, which is evident by the increased levels of serum enzymes and altered hepatic architecture.
As seen in the present study, the use of CB both as preventive and curative agent is showing the signs of the reduction in the liver damage by minimizing the micro and macro vacuoles, sinusoidal congestions and necrotic changes. However, the preventive role of CB is more effective and evident. In curative group, ten days long exposure to CCl4 would have created more damage than the capacity of the CB to overcome this damage in last five days. Therefore, alteration in the dose or extent of treatment with CB needs to be altered to see the effective counteract of induced hepatotoxicity. The hepatoprotective properties of CB extract may be due to interference of its active ingredients with cytochrome P450, resulting in the hindrance of the formation of hepatotoxic free radicals, thereby protecting the integrity of the membrane. Earlier it has been shown that phytoconstituents such as phenolics and flavonoids have significant role in protecting the liver acting as an antioxidant agents [16,17]. Therefore, the hepatoprotective activity of the CB may be attributed to its direct protection of mitochondria and strong scavenging of reactive oxygen species. In addition, methanol leaf extract of Caesalpinia crista is reported to possess DPPH radical scavenging activity thus protecting the oxidative DNA damage due to presence of phenolic and flavonoid compound [18]. As seen in the present study, the hepatoprotective activity of the CB may also due to presence of such phytoconstituents which needs to be further evaluated.
As liver is a target for many pathogens and drugs, liver toxicity is a growing medical challenge. The effective protection of liver against such toxins is a critical requirement in this modern era. Therefore, the studies like ours may through limelight on areas of cost effective alternative resources for such wonder drugs which can protect the liver damage with minimal side effects. Further, evaluation of active ingredients of CB and their mechanism of actions at cellular and molecular level is warranted and in process in our laboratory.
Effect of aqueous extracts of Caesalpinia bonduc on liver enzymes in CCl4 induced-hepatotoxic rats
| Groups | Treatment design | AST(IU /dl) | ALT ALT(IU/dl) | ALP(IU /dl) |
| I | Control | 74.5 | 40.9 | 113.26 |
| II | Tox5 | 1110.86 | 608* | 228.66 |
| III | Tox10 | 5219 | 2269 | 313 |
| VI | Preventive | 121.4 | 45.66** | 154 |
| V | Curative | 4163.67 | 1574.33 | 216.33 |
ALT- Alanine aminotransferase; AST-Aspartate aminotransferase; ALP-Alkaline phosphatase.IU/dl - International unit per deciliter.Tox5- (CCl4 0.5mg/kg, daily once for 5 Days), Tox10 - (CCl4 0.5mg/kg, daily once for 10 Days) , Preventive (CB extract once daily for 5days along with CCl4) Curative -(CCl4 for 10days followed by CB extract for last 5days). ** - when preventive group was compared with Tox5 significant reduction in the AST, ALT and ALP levels was noticed (p<0.01)
Normal liver architecture is lost when CCl4 administered for 5 days [A (10X)& A1 (40X)]. Liver showed inflammatory cell infiltration, micro and macro vacuoles in the hepatocytes, spatial degeneration of hepatocytes. However, the classical centrilobular necrosis was not evident. When both CCl4 and aqueous leaf extract of Caesalpiniabonduc administered together for 5 days, normal architecture of the liver was restored with mild inflammatory changes and micro vacuoles in the hepatocytes [B (10X)& B1(40X)]
When CCl4 alone administered for 10 days the normal architecture of the liver was more severely damaged and showed inflammatory cell infiltrations, macro vacuoles with spatial degeneration of hepatocytes and classical centrilobular necrosis (A & A1). When CCl4 is administered for 10 days along with the aqueous leaf extract of CB for the last 5 days, liver showed partial restoration of the normal architecture and inflammatory changes were seen with few lymphatic infiltrations. Hepatocytes showed few vacuoles and hepatocytes were well maintained with no signs of centriloblular necrosis (B & B1)
ALT- Alanine aminotransferase; AST-Aspartate aminotransferase; ALP-Alkaline phosphatase.IU/dl - International unit per deciliter.Tox5- (CCl4 0.5mg/kg, daily once for 5 Days), Tox10 - (CCl4 0.5mg/kg, daily once for 10 Days) , Preventive (CB extract once daily for 5days along with CCl4) Curative -(CCl4 for 10days followed by CB extract for last 5days). ** - when preventive group was compared with Tox5 significant reduction in the AST, ALT and ALP levels was noticed (p<0.01)
[1]. RK Gupta, AN Kesari, PS Murthy, R Chandra, V Tandon, G Watal, Hypoglycemic and antidiabetic effect of ethanolic extract of leaves of Annonasquamosa L. in experimental animalsJ Ethnopharmacol 2005 99(1):75-81. [Google Scholar]
[2]. Wealth of India.Raw materials, Council of scientific and industrial Research p 54-5 and volume 2, p 3-4. Publication and information directorate, New Delhi; 1950 & 1952 [Google Scholar]
[3]. NC Neogi, KP Nayak, Biological investigation ofCaesalpiniabonducella FInd J Pharmacol 1958 20:95-100. [Google Scholar]
[4]. SK Adesina, JJ Hefferren, Studies on some plants used as anticonvulsant in Amerindian and African traditional medicineFitoterapia 1982 53:147-62. [Google Scholar]
[5]. ML Dhar, MM Dhar, BN Dhawan, BN Mehrotra, C Roy, Screening of Indian plants for biological activityInd J Exp Biol 1968 6:232-47. [Google Scholar]
[6]. S Gayaraja, S Shinde, SL Agarwal, Antiasthmatic properties of Caesalpiniabonduc leavesInd J Pharmacol 1978 10:86-89. [Google Scholar]
[7]. RC Agrawal, LA Kapadia, Treatment of piles with indigenous drug-Pilex tablets and ointment alone with styplonProbe 1982 21:201-04. [Google Scholar]
[8]. K Raghunathan, R Mitra, S Karanja, Pharmacognosy of Indigenous Drugs. Part-ICentral Council for Research in Ayurveda and Siddha 1982 New Delhi, India:484-510. [Google Scholar]
[9]. JY Datte, A Traore, AM Offoumou, A Zieglera, Effect of the leaf extract of Caesalpiniabonduc on the contractile activity of uterine smooth muscle of pregnant ratJ. Ethnopharmacol 1988 60:149-55. [Google Scholar]
[10]. MN Ghosh, PJ Sheridan, Fundamentals of experimental pharmacology 1984 2nd EditionCalcuttaScientific Book Agency:669-71. [Google Scholar]
[11]. AP Manjrekar, V Jisha, PP Adhikary, MM Pai, A Hegde, M Nandini, Effect of Phyllanthusniruri Linn. treatment on liver, kidney and testes in CCl4 induced hepatotoxic ratsInd J Exp Biol 2008 46(7):514-20. [Google Scholar]
[12]. TF Slater, Biochemical mechanism of liver injuryAcademic Press 1965 [Google Scholar]
[13]. H Zimmerman, Chemical hepatic injury and its detection. In: Plaa G, Hewitt W, editors. In Toxicology of the liverJ Oral Med 1982 New YorkReven Press:1-45. [Google Scholar]
[14]. R Recknagael, Carbontetrachloride hepatotoxicityPharmacol Rev 1967 19:145-96. [Google Scholar]
[15]. SK Mitra, MV Venkataranganna, R Sundaram, S Gopumadhavan, J Ethnopharmacol 1998 63:181-86. [Google Scholar]
[16]. M Lopez, F Martinez, C Del Valle, M Ferrit, R Luque, Study of phenolic compounds as natural antioxidants by a fluorescence methodTalanta 2003 60(2-3):609-16. [Google Scholar]
[17]. CA Rice-Evans, NJ Miller, G Paganga, Structure-antioxidant activity relationships of flavonoids and phenolic acidsFree Radical Biology and Medicine 1996 20(7):933-35. [Google Scholar]
[18]. R Sarkar, B Hazra, N Manda, Hepatoprotective Potential of Caesalpinia crista against Iron-Overload-Induced Liver Toxicity in MiceEvid Based Complement Alternat Med 2012 2012:896341 [Google Scholar]