Urinary tract infection (UTI) may be defined as a condition in which bacteria are established and multiplying within the urinary tract – extending from renal cortex to urethral meatus [1]. It is caused by pathogenic invasion of the urinary tract, which leads to an inflammatory response of the urothelium leading to symptoms like burning micturation, frquency and dysuria [2]. Worldwide there are at least 150 million cases of symptomatic UTI each year, 90% of patients have cystitis and 10% pyelonephritis. The infection is sporadic in about 75% of patients and recurrent in 25%. About 2% have complicated infection [3].
The spectrum of presentation of UTI may be Asymptomatic Bacteriuria, Asymptomatic acute urethritis and cystitis, Acute pyelonephritis, Acute prostatitis, Septicemia (usually gram negative)[5].
Cephalosporins are one of the mainstays of therapy and third generation cephalosporins are the first line agents for treatment of complicated UTIs including those of nosocomial origin [6]. Cefotaxime is used in complicated urinary tract infections, lower respiratory tract infections, bacteraemia, meningitis uncomplicated gonorrhoea, infections of skin and soft tissue and of bone and joints, and obstetric and gynaecological infections [7].
Cefepime has been useful in treatment of respiratory tract infections, UTI, skin and skin structure infections and in bacteremia [8]. For infections caused by ESBL-producing E coli or Klebsiella species, Cefepime and piperacillin-tazobactam have been successful [9]. However, the indiscriminate use of third generation cephalosporins and increasing reports of bacterial resistance especially Klebsiella, Pseudomonas and many strains of E coli make it necessary to investigate new compounds.
One hundred bacteria isolates belonging to the family Enterobacteriaceae identified from different clinical specimens. These were subjected to antibiotic susceptibility testing to third-generation cephalosporins, 68% samples were resistant [10].
Cephalosporins, have a β lactam ring, which can be hydrolysed by β lactamases which by destroying the beta-lactam ring of this antibiotic class, ensures resistance [11]. One approach to counteracting this resistance mechanism has been through the development of beta-lactamase inactivators like clavulanic acid and sulbactam tazobactam, molecules with minimal antibiotic activity. However, when combined with safe and efficacious penicillins or cephalosporins, these inhibitors can serve to protect the familiar beta-lactam antibiotics from hydrolysis by penicillinases or broad-spectrum beta-lactamases [12].
Because of the wide variation in underlying abnormalities and clinical presentations, a uniform recommendation for treatment duration is likely not appropriate. Most clinical trials have evaluated 7 to 14 d of therapy, but as short as five days and as long as 20 d have been reported [13]. Keeping in view the above mentioned factors in view, the present study was designed to evaluate efficacy and tolerability of cefotaxime and sulbactam versus cefepime and tazobactam in patients of urinary tract infection.
Materials and Methods
Sixty adult patients with urinary tract infection with or without concurrent genitourinary tract pathology attending the outpatient and admitted to urology department of Rajindra Hospital, Patiala, Punjab were the subjects of this open, randomized, parallel group trial. The diagnosis was based in all of them on clinical picture and essential urine culture. Project was ethically approved by institutional ethics committee. Written informed consent was obtained from all patients. These 60 patients were randomized into two groups of 30 each and named group A and B. These were further subdivided into groups A1 and A2 and B1 and B2 depending upon the genitourinary tract (GUT) pathology, type of surgical intervention, duration of catheterization, type of sensitivity. The patients in group A1 and A2 were given Cefotaxime/Sulbactam 1.5g BD (IV/IM for 5 d and 10 d respectively). Similarly the patients in group B1 and B2 were given Cefepime/Tazobactam 1.5g BD (IV/IM for 5 d and 10 d respectively).
Inclusion criteria
Patients willing to give written consent between the ages 18-70 y will be recruited in the study. Patients with concurrent acute or chronic obstructive pathology for example benign hyperplasia of prostate will be included.
Exclusion criteria
Patients who are hypersensitive to beta-lactam antibiotics, having bleeding tendencies, abnormal renal function tests after surgical intervention and pregnant females were excluded from the study.
Investigations
Patients with signs and symptoms of UTI were subjected to the following investigations – complete urine examination, urine culture, and drug sensitivity test. Renal function tests of patients were done. Other routine investigations like Haemoglobin, TLC, DLC, were done. The treatment was started only after urine culture was found to be positive and urine culture sensitivity done.
Therapy and Follow Up
Group A1 patients were administered cefotaxime/sulbactam 1.5g BD IV/IM for 5 days and Group A2 patients were put on the same for 10 days.
Group B1 patients were given cefepime/tazobactam 1.5g BD IV/IM for 5 d and Group B2 patients were given the same for 10 days.
Initial drug choice was based on the culture sensitivity reports.
Indoor patients were examined and urine culture was regularly done on every fifth, seventh and tenth day. Postoperatively catheter was changed regularly to prevent any catheter associated UTI (CAUTI), sign and symptoms like frequency, urgency, fever were monitored during the therapy. After discharge, patients were advised follow up for any recurrence of sign and symptoms of GUT pathology (like stricture) and urinary tract infection.
Statistical Analysis
The results of the above observations of individual patients were pooled for each group. Data was compiled up and appropriate means and SD were calculated and was statistically analysed using chi square test. The results were finally displayed in tables and graphs.
Results
Patients of group A1 and B1 were assessed at day 0 (visit 1) and all findings were noted and then reassessed on day 5(visit 2).
Patients of group A2 and B2 were assessed on day 0 (visit 1), day 5 (visit 2), day 7 (visit 3) and day 10 (visit 4).
Demographic details
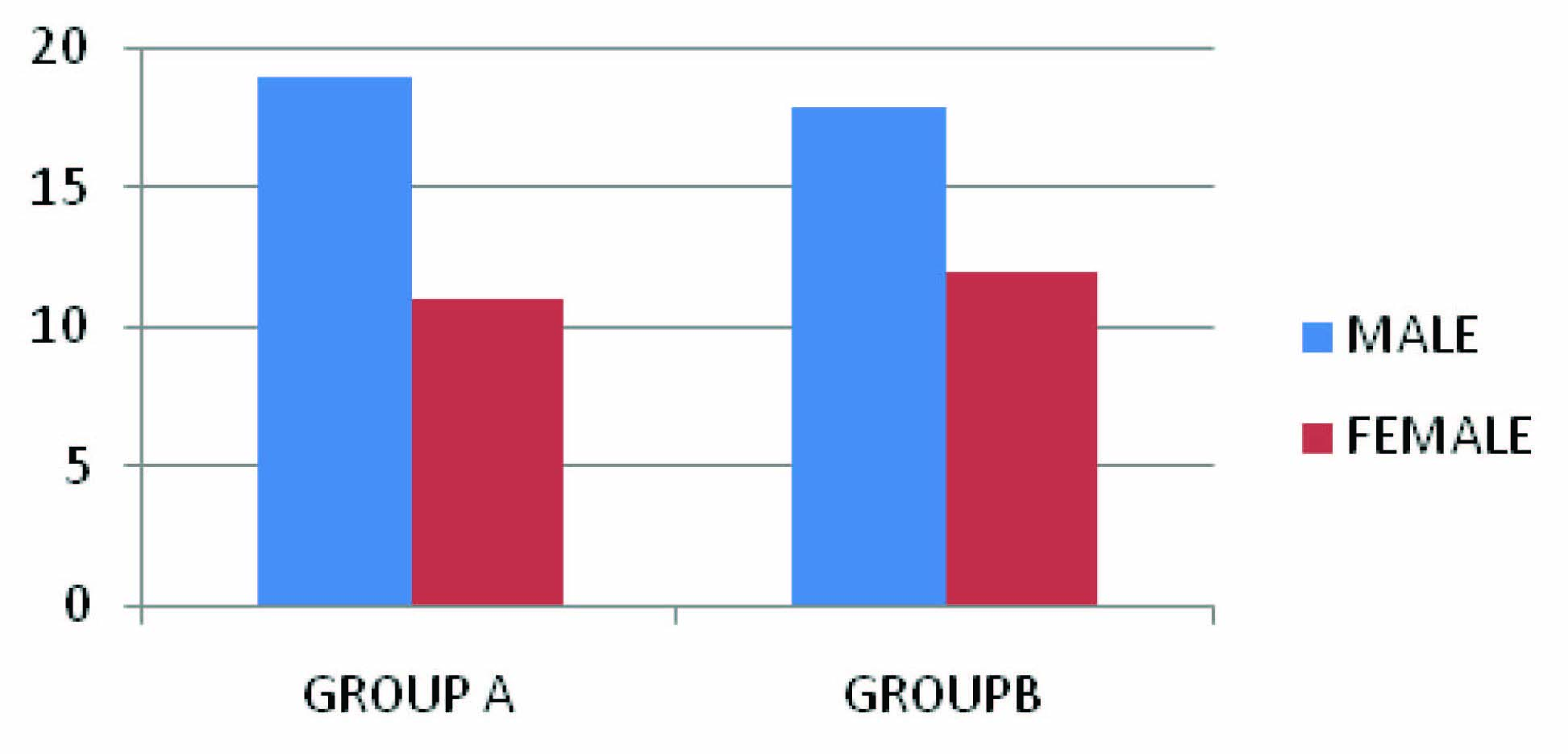
Male patients dominated in both groups as shown in [Table/Fig-1] (19 in Group A and 18 in Group B). Maximum number of patients in group A were in 51-60 y age group ,while in group B it was 61-70 y age group Minimum age was 21 y and maximum was 76 y. [Table/Fig-2].
Gender distribution., Group A: those receiving cefotaxime /sulbactam; Group B: those receiving cefepime/tazobactam
Distribution of cases according to age in both groups
| Age(in years) | Group A | Group B |
|---|
| Number | %age | Number | %age |
|---|
| 21-30 | 4 | 13% | 3 | 10% |
| 31-40 | 6 | 20% | 3 | 10% |
| 41-50 | 1 | 3% | 8 | 27% |
| 51-60 | 12 | 40% | 6 | 20% |
| 61-70 | 6 | 20% | 10 | 33% |
| 71-80 | 1 | 3% | 0 | 0% |
| total | 30 | 100% | 30 | 100% |
| Mean ±SD | 51.73 ±15.20 | 52.03± 13.87 |
Pathogen distribution and follow up
In group A, Out of 30 cases at baseline the distribution was from 23 (76%) cases E coli, 3 (10%) cases Proteus mirabilis, from 2 (7%) cases Klebsiella pneumoniae and from 2 (7%) cases Staphylococcus aureus was isolated. On visit 2 and 3 i.e. 5th day and 7th day post therapy E coli was isolated from 3 ( 10%) , Proteus mirabilis from 1 (3%) and Klebsiella pneumoniae from 1(3%) and Staph aureus were not isolated in any case. On visit 4 i.e. 10th day post therapy only one case demonstrated positivity for Klebsiella pneumonia. Rest all cases did not show any pathogen [Table/Fig-3].
Distribution of pathogens in Group A at Visit 1, 2, 3 And 4,
| Pathogens | Visit 1 | Visit 2 | Visit 3 | Visit 4 |
|---|
| No. | %Age | No. | %age | No. | %age | No. | %age |
|---|
| E. coli | 23 | 76 | 03 | 10 | 03 | 10 | Nil | Nil |
| Proteus mirabilis | 03 | 10 | 01 | 3 | 01 | 3 | Nil | Nil |
| Klebsiella pneumoniae | 02 | 7 | 01 | 3 | 01 | 3 | 01 | 3 |
| Staph aureus | 02 | 7 | Nil | Nil | Nil | Nil | Nil | Nil |
| Total cases | 30 | 100 | 05 | 16 | 05 | 16 | 1 | 3 |
day 0 = visit 1, day 5 = visit 2, day 7 =visit 3, day 10 =visit 4
Similarly distribution and follow up in group B is enumerated in [Table/Fig-4].
Distribution of pathogens in Group B at Visits 1, 2, 3 and 4,
| Pathogens | Visit 1 | Visit 2 | Visit 3 | Visit 4 |
|---|
| No. | %Age | No. | %age | No. | %age | No. | %age |
|---|
| E. coli | 21 | 70 | 1 | 3 | 1 | 3 | 1 | 3 |
| Proteus mirabilis | 1 | 3 | Nil | Nil | Nil | Nil | Nil | Nil |
| Klebsiella pneumoniae | 6 | 20 | 1 | 3 | 1 | 3 | Nil | Nil |
| Staph aureus | 2 | 7 | Nil | Nil | Nil | Nil | Nil | Nil |
| Total cases | 30 | 100 | 05 | 16 | 05 | 16 | 1 | 3 |
day 0 = visit 1, day 5 = visit 2, day 7 =visit 3, day 10 =visit 4
Bacteriological cure rates
In the present study, 5 days post therapy in group A1 and in group B1 urine culture was found to be negative in 25 and 27 out of 30 patients each i.e. the bacteriological cure rate was 80% and 86.66% respectively, as shown in [Table/Fig-5]. The cultures which was still positive were for E coli (2 patients) and Proteus (1 patient) in group A1 and for E coli (1patient) and Klebsiella (1 patient).
Comparison of urine culture between group A1 and B1
| Urine C/S | Positive | Negative | Total | %age BC | Chi square |
|---|
| Visit 1-D0 | Group A1 | 15 | Nil | 15 | | |
| %age | 100 | Nil | 100 |
| Group B1 | 15 | Nil | 15 |
| %age | 100 | Nil | 100 |
| Visit 2-D5 | Group A1 | 3 | 12 | 15 | 12 | 0.00 Y >0.05 |
| %age | 20 | 80 | 100 | 80 |
| Group B1 | 2 | 13 | 15 | 13 |
| %age | 13 | 87 | 100 | 86.66 |
BC = Bacteriological cure, Y=Yates correction; Group A1: patients receiving cefotaxime/sulbactam for 5 days; Group B1: patients receiving cefepime/ tazobactam for 5 days
10 days after treatment comparing group A2 and B2, as shown in [Table/Fig-6] at baseline, all the patients in both groups had positive urine culture, at visit 2 and visit 3, two patients in group A2 and 1 in group B2 had positive urine culture. At visit 4, one patient was still urine culture positive in group A2 while in group B2 none of the patient had a positive urine culture.
Comparison of Urine Culture in Group A2 and Group B2
| Urine C/S | Positive | Negative | Total | %age BC | Chi square |
|---|
| Visit 1-D0 | Group A2 | 15 | Nil | 15 | | |
| %age | 100 | Nil | 100 |
| Group B2 | 15 | Nil | 15 |
| %age | 100 | Nil | 100 |
| Visit 2-D5 | Group A2 | 2 | 13 | 15 | | 0.00 Y >0.05 NS |
| %age | 13 | 87 | 100 | 86.66 |
| Group B2 | 1 | 14 | 15 | |
| %age | 7 | 93 | 100 | 93.33 |
| Visit 3-D7 | Group A2 | 2 | 13 | 15 | | 0.00 Y >0.05 |
| %age | 13 | 87 | 100 | 86.66 |
| Group B2 | 1 | 14 | 15 | |
| %age | 7 | 93 | 100 | 93.33 |
| Visit 4-D10 | Group A2 | 1 | 14 | 15 | | 0.00 Y >0.05 |
| %age | 7 | 93 | 100 | 93.33 |
| Group B2 | Nil | 15 | 15 |
| %age | Nil | 100 | 100 | 100 |
BC = Bacteriological cure, Y=Yates correction; Group A2: patients receiving cefotaxime/sulbactam for 10days; Group B2: patients receiving cefepime/ tazobactam for 10 days
Comparing the two groups the bacteriological cure rate, were 93% and 100 % in the two groups at visit 4 compared to visit 1.
Statistical analysis was done and data showed the results to be non significant (>0.05).
Clinical cure rates
As evident from [Table/Fig-7] that the clinical cure rate post five days of therapy, in group A1 was 79.03%±2.82 and the same in group B1 was 87% ± 2.11.
Clinical cure rate compared in group A1 and group B1
| Duration of treatment (5 days) | Percentage of patients cured of the following symptoms | Mean±SD Overall cure rate |
|---|
| Frequency | Fever | Dysuria | Urgency | Suprapubic pain |
|---|
| Group A1 | 75 | 92.85 | 92.30 | 69.33 | 71.43 | 79.03±2.82 |
| Group B1 | 78.57 | 86.66 | 93.33 | 85.66 | 100 | 87±2.11 |
[Table/Fig-8] elucidates the clinical cure rate post ten days of therapy, in group A2 was 98.57±0.03 and the same in group B2 was 100%.
Clinical cure rate compared in group A2 and group B2
| Duration of treatment (10 days) | Percentage of patients cured of the following symptoms | Mean±SD Overall cure rate |
|---|
| Frequency | Fever | Dysuria | Urgency | Suprapubic pain |
|---|
| Group A2 | 92.85 | 100 | 100 | 100 | 100 | 98.57±0.03 |
| Group B2 | 100 | 100 | 100 | 100 | 100 | 100 |
Overall success rate of clinical improvement in the present study in group A (79.03%±2.82 + 98.57%±.03 / 200= 89.28±9.1%) i.e. those treated with cefotaxime/sulbactam was 89.28±9.1% and in group B (87% ± 2.11+ 100/200 =94.49±5.06%) i.e. those treated with cefepime/ tazobactam and 94.49±5.06% [Table/Fig-9].
| %age clinical cure rate | Chi square | ‘p’ value |
|---|
| Group A | 89.28±9.1 | 0.00 Y | 0.208 |
| Group B | 94.49±5.06 |
Adverse drug reactions
Local reactions in the form of redness, tenderness, oedema and pain at the injection sites were the most common adverse effect in both the groups. Nausea as seen in group B in 5 patients and nausea in 3 patients was the next most common effect seen. Diarrhea and headache were seen in few patients in both groups [Table/Fig-10].
Adverse drug reaction noted in both the groups
| Side effects | Group A | %age | Group B | %age |
|---|
| Local reactions | 4 | 13.33 | 6 | 20 |
| Diarrhoea | 2 | 6.66 | nil | - |
| Headache | 1 | 3.33 | 1 | 3.33 |
| Nausea | 3 | 10 | 5 | 16.66 |
Discussion
It is difficult to accurately assess the incidence of UTIs, because they are not reportable diseases. This situation is further complicated by the fact that accurate diagnosis depends on both the presence of symptoms and a positive urine culture, although in most outpatient settings this diagnosis is made without the benefit of culture [14].
Kamat et al., [15] studied epidemiology of hospital acquired urinary tract infections (HAUTI) in a medical college hospital in Goa, among 498 patients, while the overall infection rate was 8.03/100 admissions, 33.6% of the catheterized patients developed HAUTI, no overall difference in incidence in the two sexes.
The common symptom in the present study noted were increased frequency in 58 patients (96.66%). Most common affected age group was 50-70 y with male predominance. Romano and Kallis [16] similarly reported cystitis to be associated most commonly with dysuria, frequency, urgency and hematuria. Pyelonephritis was reported to be associated with fever, chills and flank pain in 306 of 352 (86.93%) patients as documented by Goffe [17].
The commonest pathogen was found to be E coli in both the groups. In lot of previous studies [14,16,17]. E coli was found as the most common pathogen causing UTI (75% of cases).
The IDSA (Infectious Disease Society of America) developed recommendations for the treatment of patients with UTIs based on a literature review that evaluated treatment regimens using 3 end points: eradication of initial bacteria, recurrence of bacteriuria, and adverse effects [18]. Because of the wide variation in underlying abnormalities and clinical presentations, a uniform recommendation for treatment duration is likely not appropriate. Most clinical trials have evaluated 7 to 14 d of therapy, but as short as five days and as long as 20 d have been reported [19].
The efficacy of third and fourth generation cephalosporin, for clinical cure, seen within their groups, were highly significant when compared in subsequent visits, and when compared for intergroup efficacy, with cefotaxime/sulbactam cure rates were 89.28%±9.1 and with cefepime/tazobactam the rate observed was 94.49%±5.06 in relieving clinical symptoms eg. fever, increased frequency, urgency, suprapubic pain and the difference in the results was statistically insignificant (p>0.05).
Cefotaxime/Sulbactam and Cefepime/Tazobactam were capable of eradicating the causative organism in 86.5%±6.5 and 93.3%±6.7 of the patients respectively, and the difference in bacteriological cure rate when compared was not statistically significant (p>0.05), however when compared intragroup at subsequent visit the results were highly significant, which indicates that cefotaxime/sulbactam is equally efficacious to cefepime/tazobactam. Combination of tazobactam and beta lactam antibiotic (ceftazidime and cefepime) it demonstrates synergistic activity (reduction in minimal inhibitory concentrations for the combination versus those of each component in a variety of organisms specially in Gram-negative Aerobes [20].
However, studies report Cefepime has the advantage of an improved spectrum of antibacterial activity, and is less susceptible to hydrolysis by some beta-lactamases, compared with third generation cephalosporins [21].
Sharifi et al., [22] conducted a study of cefepime in comparison to ceftazidime in UTI patients (total 180) and concluded cefepime produced a satisfactory clinical response in 89% of patients and eradicated 85% of pathogen in comparison to ceftazidime in which result was 86% and 78% respectively.
From the safety point of view, drugs in both the groups were well tolerated and safe, as none of the adverse effect experienced by both the patients during the trial was serious enough to lead to withdrawal of therapy.
In clinical trials using multiple doses of cefepime, 567 patients were treated with the recommended dosages of cefepime (0.5-2 g IV every 12 h). At the higher dose of 2 g every 6-8 h, the incidence of probably related adverse events was higher: rash (4%), diarrhoea (3%), nausea (2%), vomiting (1%), pruritus (1%), fever (1%) and headache [23]. Study reported by Edward et al., similarly reported no significant statistical differences in the tolerability of cefotaxime and cefepime [24].
Conclusion
The drugs in both generations of cephalosporins combined with beta lactamase inhibitors cefotaxime/sulbactam and cefepime/tazobactam were equally effective and well tolerated in the treatment of UTI, although cefotaxime/sulbactam is cost effective. However, one patient in groupA still had urine culture positive i.e. which means that in these cases the pathogens might have become resistant and did not responded to the therapy. However, long term studies in larger number of patients are required to compare efficacy, pattern of resistance shown by micro organism for these cephalosporins and to find any rare adverse effect or organ specific toxicity and safety profile of these drugs.