Background:Giardia lamblia, a flagellate protozoa, is a common causative agent of parasitic diarrhoeal diseases of humans. Laboratory diagnosis mainly consists of direct microscopic examination of stool specimen for trophozoite and cysts of Giardia. However, due to intermittent faecal excretion of parasite, the case may be miss diagnosed and the patient may continue excreting the parasite and infecting others. Therefore, other mode of diagnosis should be looked for, which overcome the above drawbacks of microscopy used alone for diagnosis.
Objectives: The present study was done to evaluate the efficacy of RIDASCREEN Giardia (ELISA) test in comparison to direct microscopy in the diagnosis of Giardia lamblia in stool specimens from patients with diarrhea and other gastrointestinal symptoms.
Materials and Methods: A total of 1680 patients were included in the study and three faecal specimens were taken from each patient which was divided into two parts. One part was used for direct wet mount examination and second part was used to put ELISA by using RIDASCREEN Giardia test.
Results: Out of 1680 stool samples, 380 specimens (22.6%) were found to be positive for Giardia lamblia. Maximum cases were detected by RIDASCREEN Giardia (ELISA) test with sensitivity of 100% and specificity of 91.5%. Maximum cases of giardiasis were detected in children less than 10 y of age (12.8%).
Conclusion: RIDASCREEN Giardia test is a rapid and effective method with high sensitivity and specificity and detects Giardia antigens in stool specimens even when the count of parasite is low, thus reducing the chances of missing even the asymptomatic cases.
Direct wet mount microscopy, Giardia lamblia, RIDASCREEN Giardia test, Stool concentration
Introduction
Giardia lamblia (also known as Giardia intestinalis and Lamblia intestinalis), a flagellate protozoa, is one of the most common pathogenic gastrointestinal parasite infecting humans [1] . It is distributed worldwide with an estimated global prevalence of 280 million cases per annum [2] . Its mode of transmission is faeco-oral due to consumption of contaminated food or water or by person to person contact in homosexuals [3] . G. lamblia infection generally causes a varied spectrum of disease ranging from the asymptomatic carrier state to acute fulminating diarrhoea or persistent diarrhoea with malabsorption in children and adults [4] . The prevalence of infection is higher in developing countries (50%, with maximum cases of 10-30% being seen in young children) due to poor hygiene and sanitary conditions. However, prevalence of giardiasis is less (1-7%) in developed countries, where it is referred as a re-emerging infectious agent, occurring in persons living in closed communities, day care nurseries, homosexual men, and in immigrants returning from highly endemic countries where it is responsible for causing traveller’s diarrhoea in 6% of patients [5,6] .
The diagnosis of giardiasis is frequently based on detection of trophozoites or cysts by microscopic examination of stool samples. However, microscopic examination of a single stool specimen has a low sensitivity (46%) due to intermittent shedding of the cysts. Hence, at least three faecal samples have to be taken and examined over a 3-5 day period to achieve 94% accuracy in diagnosing positive Giardia cases [7,8] . The main drawback of this method is that it is time and labour intensive and depends on the skill of an experienced microscopist. Also, the parasite may be hidden by bile pigments and not visualized by wet mount examination [9,10] .
The string test, duodenal aspirate, intestinal impression smear and intestinal biopsy have all been proposed as techniques to improve Ahmad3the conventional microscopic diagnosis. However, these are invasive and more expensive tests with little diagnostic gain [11] .
Now a days, immunological diagnosis can be done based on detection of anti-Giardia antibodies and Giardia antigens in stool specimens [12,13] However, there is presence of poor correlation between positive anti-Giardia antibody titers and the presence of active Giardia infection [14] . But, this problem has been overcome in direct detection of Giardia lamblia antigen in stool (Copro-antigen diagnosis) which was initially done by using counterimmunoelectrophoresis [15] . Now a days, the most widely used antigen detection immunoassays for Giardia are the direct fluorescent-antibody (DFA) tests which detect intact organisms, and enzyme immunoassays (EIAs) which detect soluble stool antigens [16,17] . The advantages of the EIA over DFA are that numerous samples can be screened at one time, and secondly the tests can be read objectively on a spectrophotometer instead of subjectively on a fluorescence microscope [18] .
The recently licensed commercially available enzyme-linked immunosorbent assay (ELISA) kits are found to be rapid and effective methods to diagnose Giardia infection in patients with gastrointestinal symptomatology by detecting Giardia lamblia associated antigens in stool. Hence, the present study was done to evaluate the efficacy of an FDA approved commercial ELISA kit, RIDASCREEN Giardia test (R-Biopharm AG, Darmstadt, Germany) for diagnosing intestinal giardiasis and to compare it with direct microscopic examination of stool samples of patients with gastrointestinal symptoms.
Materials and Methods
A total of 1680 patients were included in the study that came to Era’s Lucknow Medical College and Hospital with complaints of diarrhoea and other gastrointestinal symptoms. The study was conducted over a period of one year (from March 2011 to February 2012). An oral informed consent was taken from each patient before collection of specimen.
Specimen collection
Three stool specimens (free from urine) were obtained from each patient on every other day and collected in a 25 ml wide mouth plastic universal container without preservatives [19] . Each sample was divided into 2 parts. First part was used to prepare slides for direct wet mount examination and slides prepared after concentration methods. Second part was immediately stored at -20°C for performing ELISA later.
Direct wet mount Microscopy
For each sample, normal saline mount and iodine mount were prepared on the same slide and examined microscopically at 10X and 40X for the presence of Giardia lamblia> trophozoites and cysts [19,20].
Formalin-ether sedimentation
Another iodine wet mount was prepared after concentrating the faecal specimen by Formalin-ether sedimentation method as per CLSI guideline [19] . The prepared mount was observed at 10X and 40X of light microscope and the cysts of Giardia were identified by their characteristic morphology.
ELISA by RIDASCREEN Giardia test
Principle
The RIDASCREEN Gardia test (R-Biopharm AG, Darmstadt, Germany) is an enzyme immunoassay based on the detection of antigens of Giardia lamblia cysts and trophozoites in stool specimen. Here, Giardia-specific antibody is coated on the surface of the well of microtitre plate. Then stool sample is added followed by addition of conjugate. If Giardia lamblia is present in the specimen, a sandwich complex forms which is made up of the immobilized antibodies, the Giardia lamblia antigens and the conjugated antibodies. Unattached enzyme-labelled antibodies are removed during the washing phase. In positive test, on addition of the substrate, there is a change in the colour of the well of the microtitre plate from colourless to blue. On adding the stop reagent, the colour changes from blue to yellow.
Procedure
The test was performed according to manufacturer’s instructions (R-Biopharm AG, Darmstadt, Germany). The thawed stool samples (100 mg) were mixed with 1 ml of sample dilution buffer and centrifuged at 5000 rpm for 5 min. The supernatant was taken for further tests. 100 μl of stool suspensions were pipetted in the microwells along with 100 μl each of positive and negative controls provided by the manufacturer. Then 100 μl of enzyme-conjugated antibody was added, mixed thoroughly and incubated at room temperature (20-25°C) for 60 min. The wells were washed 5 times with 300 μl of wash buffer each time. After washing for the last time, the plate was knocked out thoroughly onto a clean absorbent paper in order to remove any residual moisture. Then 100 μl of substrate was added to each well and the plate was incubated at room temperature (20-25°C) in the dark for 15 min. Then, 50 μl of stop reagent was added to each well and mixed properly. The absorbance of controls and patient samples was read at 450 nm using an ELISA micro-titre plate reader (Immunoskan-MS, Biological Diagnostic Supplies Limited, UK).
Results
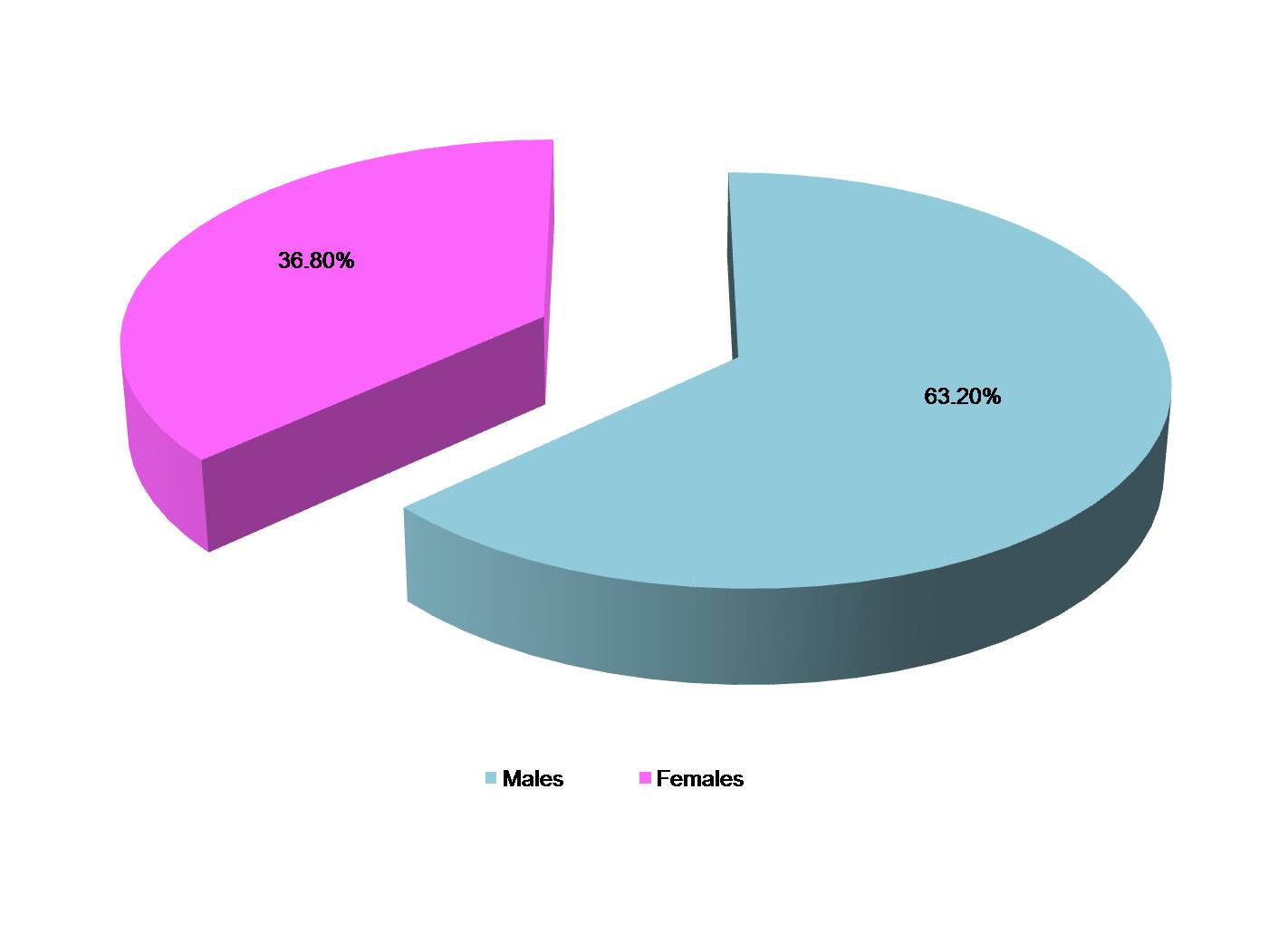
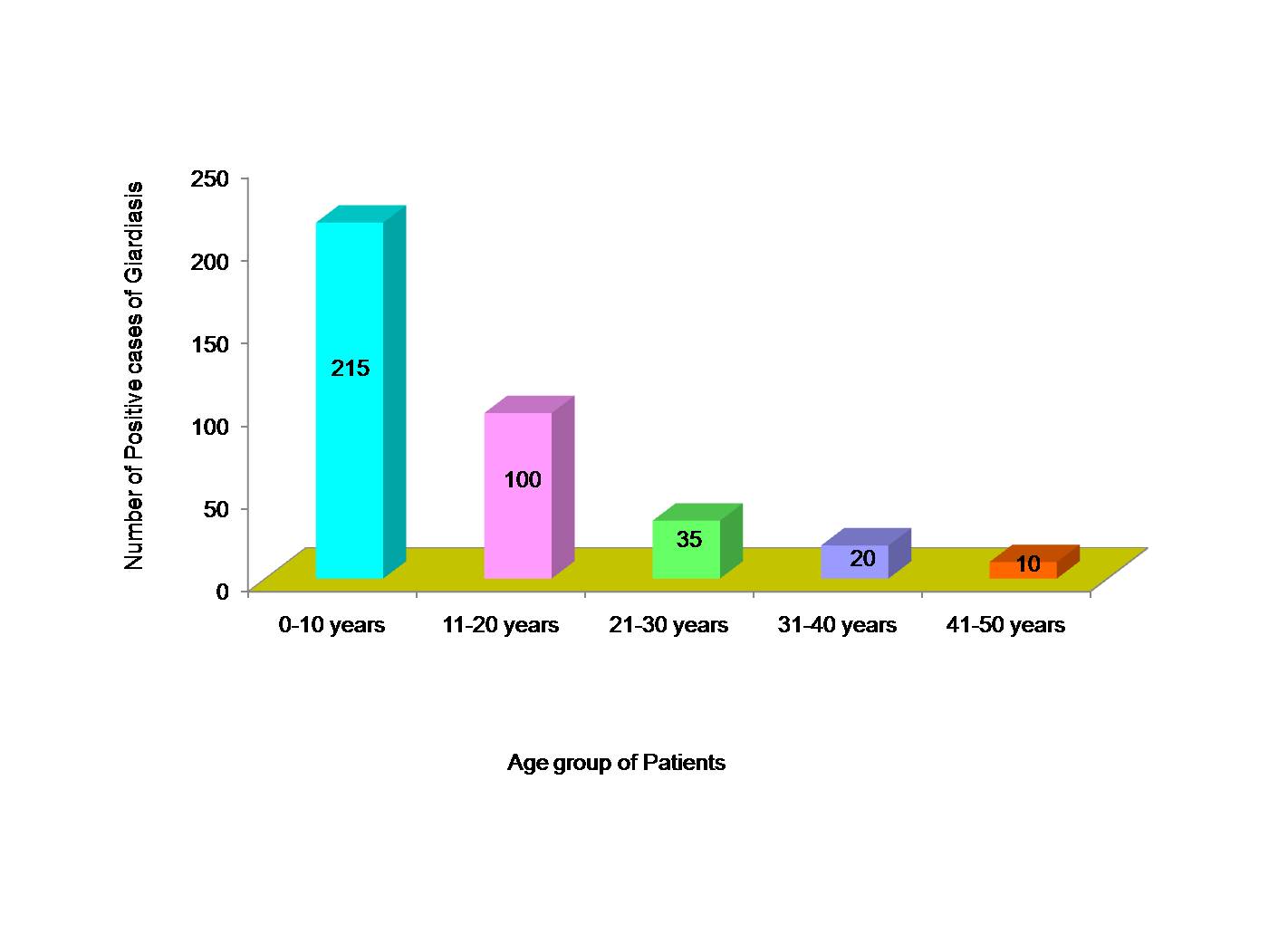
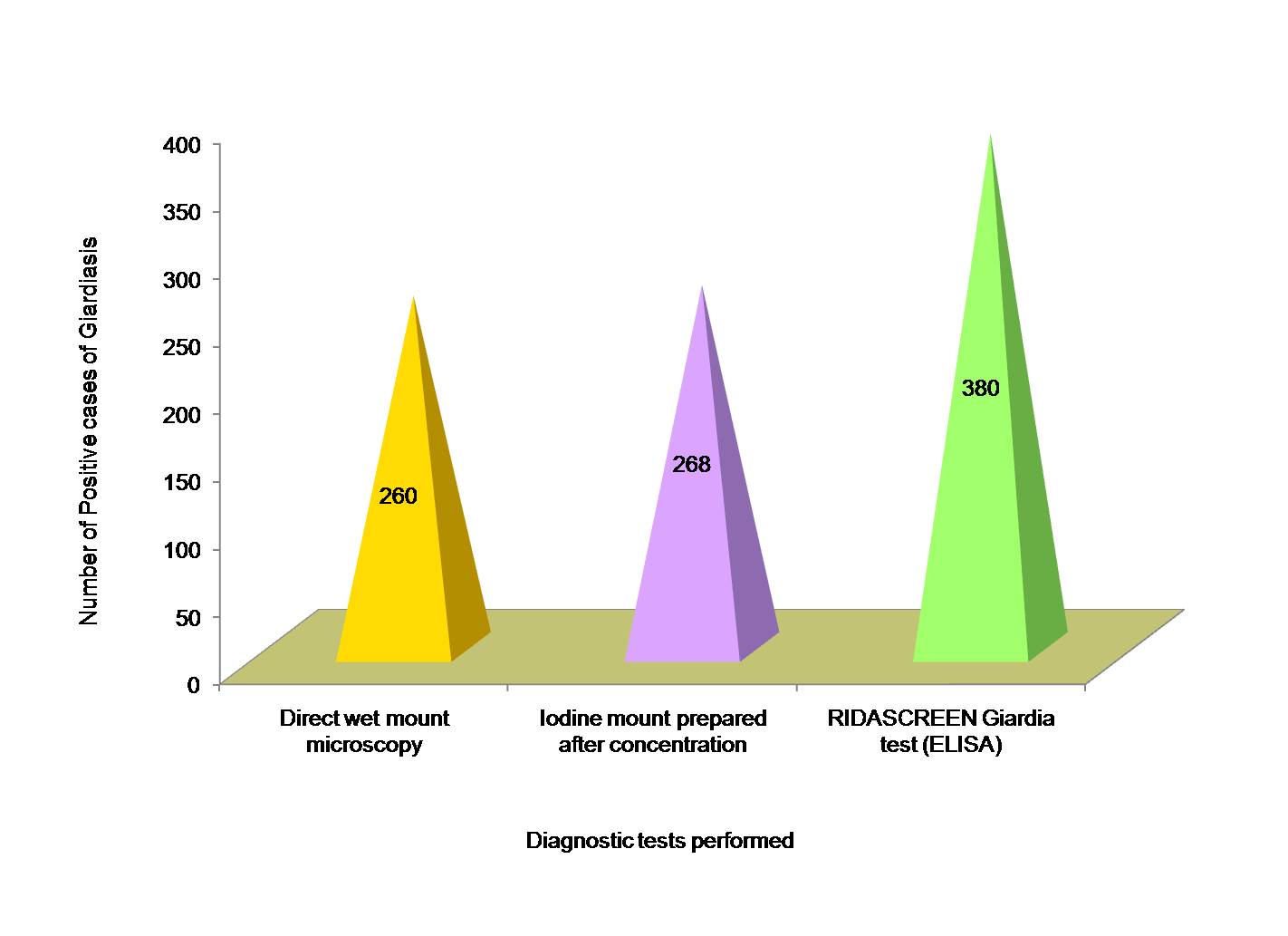
Out of 1680 faecal specimens tested, 380 specimens (22.6%) were found to be positive for Giardia lamblia. Out of 380 cases of giardiasis, 240 were males and 140 were females [Table/Fig-1]. Age of patients ranged from 3-45 y and mean age was 18.56 ± 0.34 y. Maximum cases of giardiasis as diagnosed by ELISA test were detected in children of < 10 y of age (12.8%), followed by 11-20 y age group (6.0%) and least number of cases (0.6%) in 41-50 y age group [Table/Fig-2]. Amongst the positive cases of Giardia lamblia, maximum were detected by RIDASCREEN Giardia (ELISA) test (22.6%) followed by microscopic slide prepared after formalin-ether concentration technique (16.0%) and direct wet mount examination (15.5%) as shown in [Table/Fig-3]. A comparison between microscopic stool examination (gold standard) and ELISA test for diagnosis of Giardia lamblia was done and sensitivity and specificity of RIDASCREEN Giardia test was calculated [Table/Fig-4].
Discussion
Giardia lamblia is a common parasitic cause of diarrhoea particularly in children leading to malnutrition and stunted growth of the affected child. Laboratory diagnosis is frequently based on detection of cysts and trophozoites of the parasite by direct wet mount microscopy. Although conventional microscopy of three stool samples (with or without concentration techniques) is still being recommended as “gold standard” to diagnose infections caused by G lamblia, but its sensitivity is found to be low (50-70%) even after multiple examinations [21]. Now a days, antigen detection assays for G lamblia have proven to be very useful with the advantages of reduced labor and time consumption required in its diagnosis. ELISA with sensitivity of 95% to 100% and specificity over 90% when compared with direct microscopy provides a relevant alternative method to the routine ova and parasite (O & P) examination method in diagnosing giardiasis with the added advantage of increased sensitivity required to confirm infections in patients with low parasite numbers and diagnose the disease even if the live parasite is absent in the faecal samples [21-24].
RIDASCREEN Giardia test is a recent FDA approved enzyme linked immunosorbent assay which detects Giardia lamblia antigen in stool samples. In our study, out of 1680 patients, 380 were diagnosed positive for Giardia lamblia infection by RIDASCREEN Giardia (ELISA) test which was far better than direct wet mount microscopy which detected only 260 positive cases of giardiasis. The sensitivity and specificity of ELISA test in comparison with direct wet mount microscopy was found to be 100% and 91.5% respectively. This is comparable to another study which showed sensitivity and specificity of ELISA test in comparison to microscopy as 98.8% and 100% respectively [2]. In another study sensitivity and specificity of ELISA test was found to be 76.4% and 100% respectively [20]. This reduced sensitivity could be due to less number of samples included in their study, as the sensitivity of ELISA test has been found to improve with increase in the number of specimens [22].
In the present study, out of the 380 positive cases, maximum cases of giardiasis were detected in children less than 10 y of age (12.8%). Another study also detected maximum cases of positive infection with G lamblia in children less than 18 y of age by ELISA test [2]
Distribution of patients suffering from intestinal giardiasis according to sex
Age distribution of patients with intestinal giardiasis as diagnosed by RIDASCREEN Giardia test
Detection of Giardia lamblia in stool specimens by different diagnostic tests
Comparison of efficacy of Enzyme Linked Immunosorbent Assay test taking direct microscopy as gold standard
| ELISA | Microscopy | Total stool samples tested | Sensitivity of ELISA (%) | Specificity of ELISA (%) | Positive Predictive Value of ELISA (%) | Negative Predictive Value of ELISA (%) |
|---|
| Positive | Negative |
|---|
| Positive | 260 | 120 | 380 | 100 | 91.5 | 68.4 | 100 |
| Negative | 0 | 1300 | 1300 |
| Total stool samples tested | 260 | 1420 | 1680 |
Conclusion
Although, direct wet mount microscopy is considered as gold standard for diagnosis of giardiasis, but it may give false negative results, especially, in chronic infection due to intermittent shedding of cysts. This problem is overcome by enzyme immunoassays which detects Giardia antigen in stool specimens. RIDASCREEN Giardia test is easier to perform and is useful for rapid investigation of large number of stool specimens. Thus, it may help in early diagnosis and prompt treatment of children infected with Giardia, thereby improving their physical growth and development and preventing adverse sequelae in the form of chronic giardiasis.
[1]. SP Johnston, MM Ballard, MJ Beach, L Causer, PP Wilkins, Evaluation of Three Commercial Assays for Detection of Giardia and Cryptosporidium Organisms in Fecal SpecimensWilkinsJ Clin Microbiol 2003 41(2):623-26. [Google Scholar]
[2]. B Chakarova, Comparative evaluation of the diagnostic methods for detection of Giardia intestinalis in human fecal samplesTrakia Journal of Sciences 2010 8(S2):174-79. [Google Scholar]
[3]. MA Behr, E Kokoskin, TW Gyorkos, L Cedilotte, GM Faubert, JD Maclean, Laboratory diagnosis of Giardia lamblia infection: A comparison of microscopy, coprodiagnosis and serologyCan J Infect Dis 1997 8(1):33-38. [Google Scholar]
[4]. P Dutt, S Mehta, VK Vinayak, Enzyme linked immunosorbent assay for coprodiagnosis of giardiasis and characterization of a specific Giardia lamblia antigen in stools J Med Microbiol 1991 34:271-75. [Google Scholar]
[5]. National Science and Technology Council: Infectious disease – a global health (report of the National Science and Technology Council on emerging and reemerging infectious diseases), Office of Science and Technology Policy, Washington, DC, USA, 1995 [Google Scholar]
[6]. SA Ali, DR Hill, Giardia intestinalisCurr Opin Infect Dis 2003 16:453-60. [Google Scholar]
[7]. KL Hanson, CP Cartwright, Use of an enzyme immunoassay does not eliminate the need to analyze multiple stool specimens for sensitive detection of Giardia lambliaJ Clin Microbiol 2001 39:474-77. [Google Scholar]
[8]. T Weitzel, S Dittrich, I Mohl, E Adusu, T Jelinek, Evaluation of seven commercial antigen detection tests for Giardia and Cryptosporidium in stool samplesClin Microbiol Infect 2006 12:656-59. [Google Scholar]
[9]. EW Koneman, SD Allen, WM Janda, PC Schreckenberger, WC Winn, Diagnostic MicrobiologyJB Lippincott Company 1992 :901 [Google Scholar]
[10]. MJ Gharavi, A Fallahi, B Qara-gozlou, Evaluation of Giardia detection by routine parasitical assays and antigen detection techniquesJ Periodontol 2005 5:346-49. [Google Scholar]
[11]. A Paerregaard, K Hjelt, Comparative study of four methods for detecting giardiasis in childrenPediatr Infect Dis 1988 7:807-09. [Google Scholar]
[12]. PD Smith, FD Gillin, WR Brown, TE Nash, IgG antibody to Giardia lamblia detected by enzyme-linked immunosorbent assayGastroenterology 1981 80:476-80. [Google Scholar]
[13]. VK Vinayak, K Kum, R Chandna, K Venkateswarlu, S Mehta, Detection of Giardia lamblia antigen in the feces by counterimmunoelectrophoresisPediatr Infect Dis J 1985 4:383-86. [Google Scholar]
[14]. JD Rosoff, CA Sanders, SS Sonnad, PR De Lay, WK Hadley, FF Vincenzi, Stool diagnosis of giardiasis using a commercially available enzyme immunoassay to detect Giardia specific antigen 65 (GSA 65)J Clin Microbiol 1989 27:1997-2002. [Google Scholar]
[15]. JC Craft, JD Nelson, Diagnosis of giardiasis by counter-immunoelectrophoresis of fecesJ Infect Dis 1982 145:499-504. [Google Scholar]
[16]. LS Garcia, AC Shum, DA Bruckner, Evaluation of a new monoclonal antibody combination reagent for direct fluorescence of giardia cysts and cryptosporidium oocysts in human fecal specimensJ Clin Microbiol 1992 30:3255-57. [Google Scholar]
[17]. LS Garcia, RY Shimizu, Detection of Giardia lamblia and Cryptosporidium parvum antigens in human fecal specimens using the ColorPAC combination rapid solid-phase qualitative immunochromatographic assayJ Clin Microbiol 2000 38(3):1267-68. [Google Scholar]
[18]. LS Garcia, RY Shimizu, Evaluation of nine immunoassay kits (enzyme immunoassay and direct fluorescence) for detection of Giardia lamblia and Cryptosporidium parvum in human fecal specimensJ Clin Microbiol 1997 35:1526-29. [Google Scholar]
[19]. Clinical and Laboratory Standards Institute (CLSI). Procedures for the recovery and identification of parasites from the intestinal tract; Approved Guideline— Second Edition. CLSI document M28-A2. Clinical and Laboratory Standards Institute, Wayne, Pennsylvania, USA 2005 [Google Scholar]
[20]. AT Al-Saeed, SH Issa, Detection of Giardia lamblia antigen in stool specimens using enzyme-linked immunosorbent assay Eastern Mediterranean Health Journal 2010 16(4):362-64. [Google Scholar]
[21]. A Barazesh, J Majidi, E Fallah, R Jamali, J Abdolalizade, R Gholikhani, Designing of enzyme linked immunosorbent assay (ELISA) kit for diagnosis copro-antigens of Giardia lambliaAfri J Biotechnol 2010 9(31):5025-27. [Google Scholar]
[22]. DG Addiss, HM Mathews, JM Stewart, SP Wahlquist, RM Williams, RJ Finton, Evaluation of a commercially available Enzyme-linked immunosorbent assay for Giardia lamblia antigen in stoolJ Clin Microbiol 1991 29(6):1137-42. [Google Scholar]
[23]. CL Chappell, CC Matson, Giardia antigen detection in patients with chronic gastrointestinal disturbances J Fam Pract 1992 35:49-53. [Google Scholar]
[24]. T Jelinek, S Neifer, Detection of Giardia lamblia stool samples: a comparison of two enzyme-linked immunosorbent assaysF1000 Research 2013 2:39-44. [Google Scholar]