Oral cancer is one of the most common cancers in the world. Despite optimal and radical surgical treatment, the prognosis of this cancer still remains poor. Carcinomas are composed of diverse, heterogeneous cell populations that are different in their characteristics. Although histopathological grading is often considered as a tool to judge the aggressive behavior of the carcinoma, it is not possible to identify cells which have capacity to metastasize [1–4].
It is firmly established that the tumour releases many angiogenic factors responsible for activation of endothelial cells to grow towards developing tumour. A substantial body of work indicates that increased micro vessel density (MVD) is associated with advanced tumour stage, metastasis, and a poor prognosis [5].
Compared with blood vessels, the lymphatic system has been relatively poorly studied, because previously there was lack of specific antibodies. Erroneously, lymphatic vessels were also included during analysis of blood vessels. Recently, with the growing knowledge of lymphangiogenesis and specific lymphatic vessel markers, we have considerable evidences suggesting that tumour lymphangiogenesis (formation of new lymphatic vessels) plays an important role in lymph node metastasis [6]. Furthermore, recent studies suggested the existence of a lymphatic system within the inferior alveolar canal, pulp and periodontal tissues which may further contribute in loco-regional metastasis [7].
Today we have several relatively specific antibodies for lymphatic endothelium, such as VEGFR3, podoplanin, LYVE-1; D2-40 and Prox l [6]. In the present study, D2-40 was used to examine lymphangiogenesis separately and more objectively from angiogenesis. The lymphatic vessel density (LVD) and micro vessel density (MVD) were immunohistochemically measured with an attempt to correlate them in different histopathological grades and in intratumoural and peritumoural areas of OSCC.
Materials and Methods
Samples
Forty two formalin-fixed paraffin-embedded (FFPE) tissue blocks of excisional biopsy specimens of OSCC treated between January 2008 to December 2011 were retrieved from the archives of Department of Oral and Maxillofacial Pathology, PMNM Dental College & Hospital, Bagalkot – Karnataka, India after the approval of the Institute’s research ethics committee. One FFPE tissue block of lymphangioma was included as a positive control and one squamous cell carcinoma tissue with exclusion of primary antibody was used as negative control.
Tumours were graded according to WHO grading system (Modified Broder’s System) [4] into well- {WDSCC (Grade I); n = 20}, moderately {MDSCC (Grade II); n = 12}, and poorly differentiated {PDSCC (Grade III); n = 10 }.
Immunohistochemical Procedure
Histology and immunohistochemistry were performed on sections of 4μm thickness from formalin-fixed, paraffin-embedded tissues, dewaxed through xylene and graded concentrations of ethanol. For histology, sections were stained with hematoxylin and eosin (H&E). For immunohistochemistry, the sections were mounted on the 3-aminopropyl triethoxy silane coated glass slides and were deparaffinized in xylene for 30 minutes and slides were hydrated through graded iso-propyl alcohol 100%, 95%, 70%, 50% and washed under tap water. Microwave antigen retrieval was done using citrate buffer {pH-6.0} at 800 W for 10 min, 420 W for 10min and 360 W for 5 min and solution was allowed to cool to room temperature. Then, the sections were washed in Tris Buffered Saline (TBS) having pH 7.4 for 10 min and incubated with 3% H2O2 in menthol for 5 min at room temperature to block the endogenous peroxidase activity. Then washed in TBS for 10 min and incubated in mouse serum for 10 min to block the non-specific staining (background staining). Sections were then incubated with primary antibody CD34 and antibody D2-40 on the consecutive sections for 30 min at room temperature. Then washed in TBS for 10 min and then incubated with super enhancer for 10 min. Washed in TBS for 10 min and incubated with poly horse radish peroxidase for 30 min. Then again washed in TBS for 10 minutes and then incubated with freshly prepared substrate DAB chromogen for 5-10 min (DAB chromogen was prepared by adding 20 μl of DAB to 1 ml of buffer substrate). Then washed for 10 min in TBS to stop chromogen reaction and then washed in tap water for 10-20 min. The slides were then dehydrated using graded isopropyl alcohol and cleared using 3 changes of xylene for 10 min and mounted with DPX.
Assessment
Micro vessel density (CD34 positivity) and lymphatic vessel density (D2-40 positivity) in both intratumoural and peritumoural areas were assessed by hot spot method [8,9]. The immunohistochemical positive reaction of D2-40 and CD34 antibodies was evaluated considering its expression in the cytoplasm of lymphatic vessel and blood vessel endothelial cells, respectively [10]. Any brown staining of endothelial cell or endothelial cell cluster that was clearly separate from adjacent vessels, tumour cells, and other connective tissue elements was considered a single, countable vessel. Vessel lumen was not considered to be defined as a micro vessel or lymphatic vessel, and red cells were not used to define a blood vessel. Vessels within the main tumour mass, surrounded by tumour cells were considered as intratumoural. Vessels within one high-power field from the tumour border were considered as peritumoural. Vessels more than one high-power field away from the invasive tumour front were not counted [6].
All the slides were evaluated by two experienced oral pathologists to avoid inter-observer bias.
Statistical Analysis
The statistical significance of differences in data for LVD and MVD between groups was determined by one-way-ANOVA test, Tukey’s multiple post hoc procedure and t-test. A value of P<0.05 was considered statistically significant.
Results
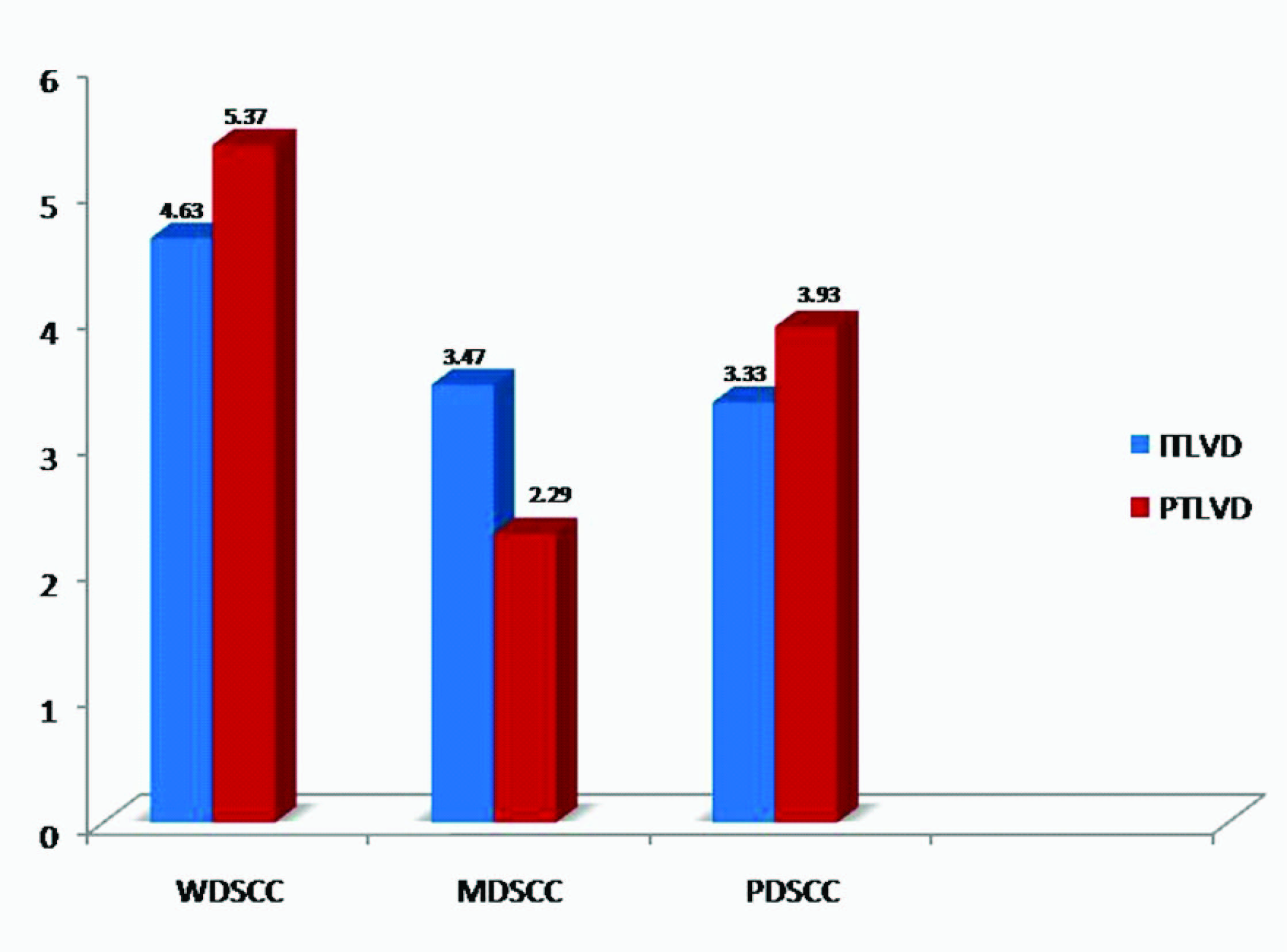
Localization of lymphatic vessels: D2-40 positivity was present in all cases. Peri-tumoural lymphatic vessel density (PTLVD) was found to be significantly more in WDSCC than MDSCC (p= 0.0232) [Table/Fig-1]. The mean ITLVD & PTLVD was found highest in WDSCC [Table/Fig-2].
Comparison of mean values of PTLVD in different histopathological grades by Tukeys multiple post hoc procedures
| Groups | WDSCC | MDSCC | PDSCC |
|---|
| Mean | 5.3704 | 2.9444 | 3.9333 |
| WDSCC | - | | |
| MDSCC | p=0.0232* | - | |
| PDSCC | p=0.2524 | p=0.5588 | - |
*p<0.05- statistically significant
Comparison of ITLVD and PTLVD in different grades
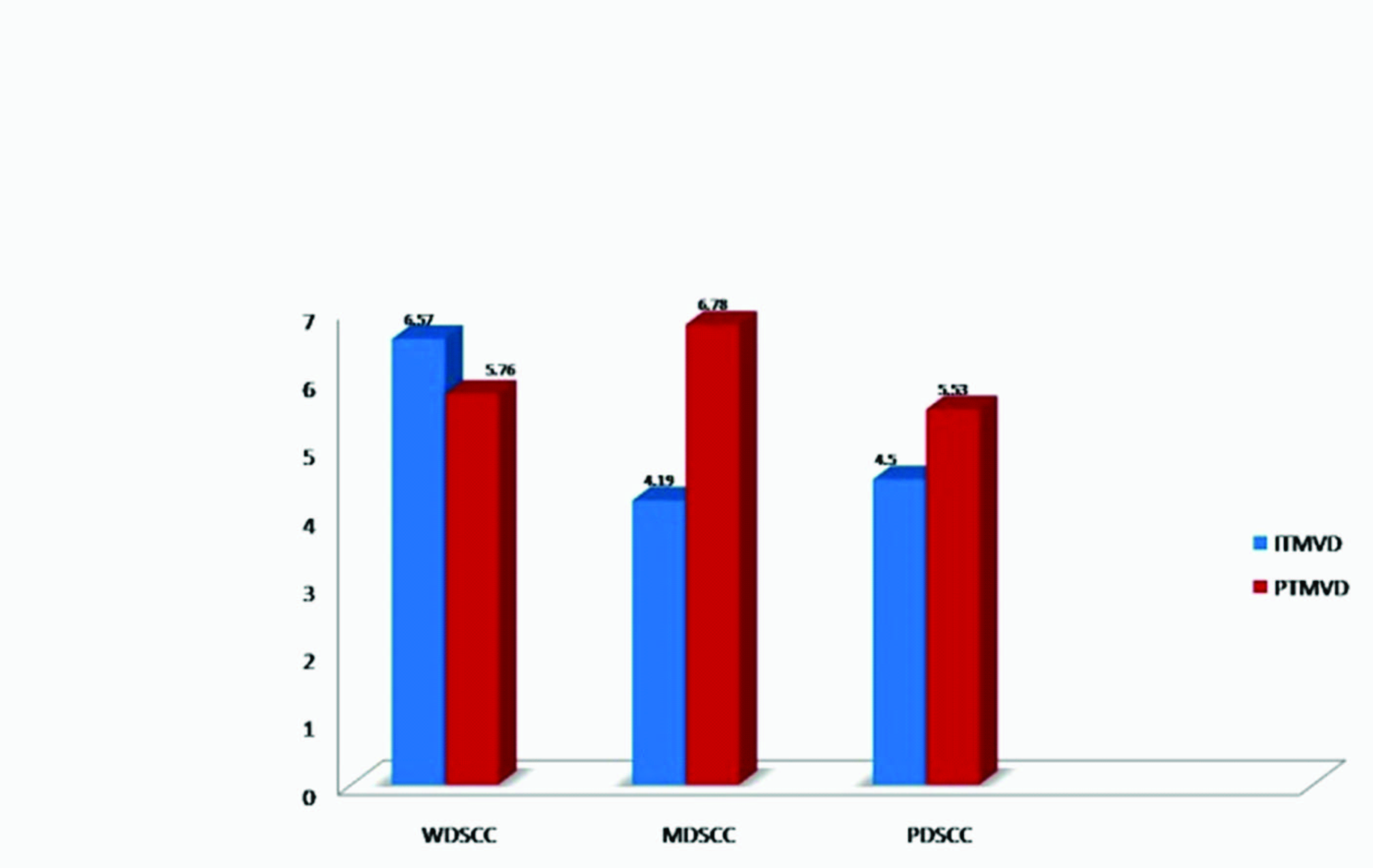
Localization of blood vessels: CD34 positivity was present in all cases. In MDSCC, Peritumoural micro vessel density (PTMVD) was found to be significantly more than intratumoural micro vessel density (ITMVD) (p<0.05) [Table/Fig-3]. The mean ITMVD was found highest in WDSCC while the mean PTMVD was found to be highest in MDSCC [Table/Fig-4].
Comparison of intratumoural and peritumoural vessels (both MVD and LVD) in different grades by t-test
| Positivity | Intra-Tumoural | Peri-Tumoural | p-value |
|---|
| Mean | Mean |
|---|
| WDSCC (CD 34 positivity- MVD) | 6.5741 | 5.7593 | 0.6518 |
| WDSCC (D2-40 positivity- LVD) | 4.6296 | 5.3704 | 0.3671 |
| MDSCC (CD 34 positivity- MVD) | 4.1944 | 6.7778 | 0.0496* |
| MDSCC (D2-40 positivity- LVD) | 3.4722 | 2.9444 | 0.5772 |
| PDSCC (CD 34 positivity- MVD) | 4.5000 | 5.5333 | 0.4202 |
| PDSCC (D2-40 positivity- LVD) | 3.3333 | 3.9333 | 0.4908 |
Comparison of ITMVD and PTMVD in different grades
Overall, PTLVD was found greater than intra-tumoural lymphatic vessel density (ITLVD) irrespective of grade of tumour, but the results were not statistically significant (p>0.05). Similarly, overall PTMVD was found greater than ITMVD irrespective of grade of tumour, but the results were not statistically significant (p>0.05) [Table/Fig-5].
Comparison of intratumoural and peritumoural vessels (MVD and LVD) by t-test (In total samples, regardless of grades)
| Positivity | Intra-Tumoural | Peri-Tumoural | p-value |
|---|
| Mean | Mean |
|---|
| CD 34 positivity | 5.3417 | 6.0083 | 0.4761 |
| D2-40 positivity | 3.9583 | 4.2833 | 0.5569 |
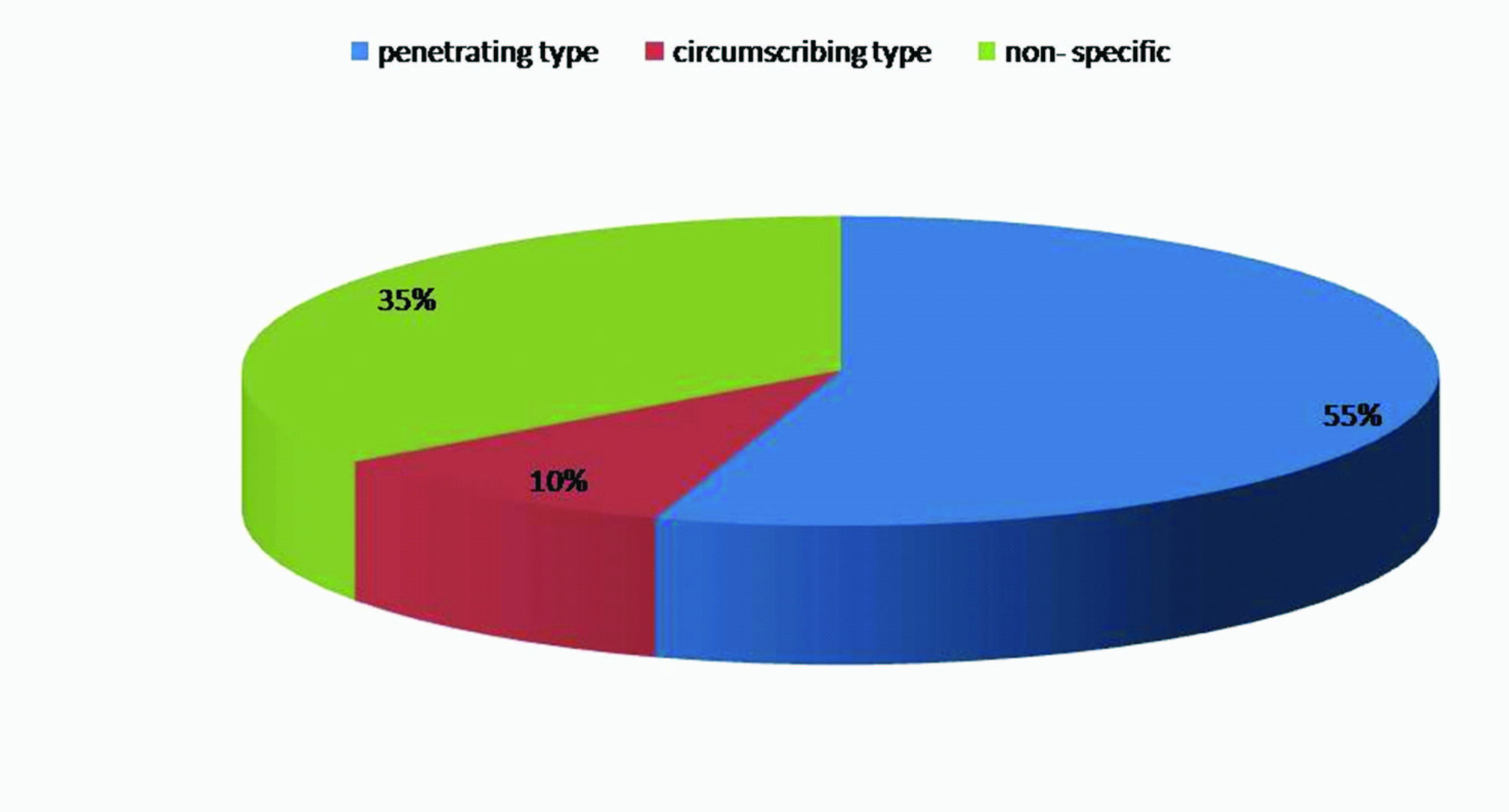
Patterns of distribution of CD34 positive vessels: Two patterns of distribution of CD34 positive vessel were observed in the cancer nest area. The first pattern, designated as circumscribing type, observed in four cases. The second pattern, designated as penetrating type, observed in 22 cases. In remaining cases, distribution of micro vessels was non-specific [Table/Fig-6].
Distribution of CD34 positive vessels pattern in cancer nest
Discussion
The proliferation, invasion and metastasis of many cancers are angiogenic dependent; they require neovascularization to grow beyond 1mm [3]. The degree of neovascularization can be measured by micro vessel density. This method was developed by Weidner et al., by using antibodies against factor VIII-related antigen that stains mainly mature vessels. Recent antibodies used against CD34 react not only with newly formed vessels but also with normal vessels trapped within tumour tissues and are thus referred to as pan endothelial markers, which has been used in the present study [11,12].
For OSCC, analysis of lymphatic spread is more important than in other tumours because they preferentially metastasize to lymph nodes. During the progression of OSCC, lymph vessels are repeatedly destroyed and regenerated with the invasion of cancer cells. Studies have shown that lymphangiogenesis was closely associated with an increased risk of lymph node metastasis. It is unclear; however, whether this is a consequence of intratumoural lymphatics or peritumoural lymphatics or both. Few studies support peritumoural lymphatics as a major drainage channel for cancer cells but others suggest a significant relationship between the presence of intratumoural lymphatics and nodal metastases [13].
Differentiation between lymphatics and blood vessels by light microscopy has long been a problem, since differentiation criteria for these structures are inevitably subjective. Fortunately, several specific antibodies for lymphatics have been found in recent years, which include VGFR-3, podoplanin, Prox1, LYVE-1 and D2-40 monoclonal antibody [9]. The monoclonal antibody D2-40 was initially developed to recognize the M2A antigen, which is an oncofetal glycoprotein expressed by testicular germ cell neoplasm. Because M2A was found in lymphatic endothelial cells but not blood endothelial cells, D2-40 has been used to detect lymphatic vessels in the present study. Here, D2-40 expression was consistently detected in endothelial cells of lymphatic vessels, but was variable with the frequent staining of tumour cells also [Table/Fig-7 & 8]. We used lymphangioma as a positive control in which lymphatic endothelium showed positivity for D2-40 but was negative for CD34, affirming its specificity as a lymphatic marker [Table/Fig-7a & 8a].
(a) D2-40 positive expression in lymphangioma (positive control)
(b) D2-40 positive intratumoural lymphatic vessels
(c) D2-40 positive peritumoural lymphatic vessels
(d) Diffuse Pattern of D2-40 Positive expression pattern by tumour cells
(e) Focal Pattern of D2-40 Positive expression pattern by tumour cells
(f) D2-40 expression in hyperplastic epithelium adjacent to carcinoma
(g) D2-40 positive expression in stroma
(h) D2-40 positive expression in salivary gland ducts & myoepithelial cells

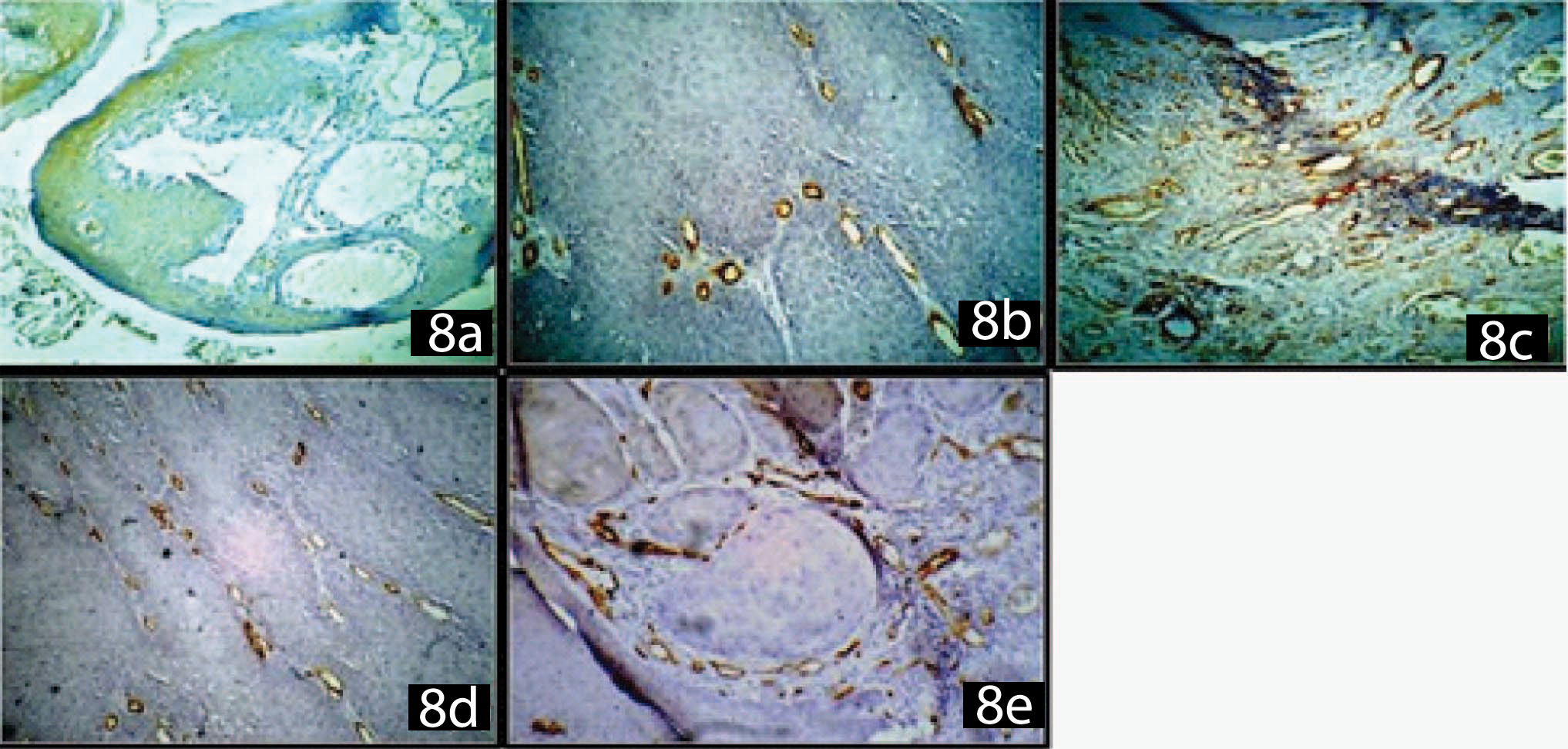
(a) CD34 negative expression in lymphangioma
(b) CD34 positive intratumoural micro vessels
(c) CD34 positive peritumoural micro vessels
(d) CD 34 positive vessels: Penetrating Type
(e) CD34 positive vessels: Circumscription Type
We found mean ITLVD and PTLVD highest in WDSCC. This may be attributed to differences in patient selection, methodology, the types of tumours, different anatomic origin included in the analysis [6]. Further comparison of D2-40 positive vessels in intra-tumoural (IT) and peri-tumoural (PT) area of OSCC revealed high LVD in PT area than IT area regardless of the grade of OSCC. The reason could be that, the intra-tumoural lymphatic vessels were pre-existing, entrapped from the normal tissue, rather than proliferating vessels. The other possibility could be that VEGF-C expression was down regulated in the intra tumoural tissue, especially in the deeper area near the invasive front. Alternatively, it can be speculated that a natural inhibitor of lymphangiogenesis may be present in the tumour stroma [14].
Interestingly, similar to Yuan P et al., [15], we also found that apart from lymphatic vessels, D2-40 was also expressed by tumour cells, which made counting of intratumoural lymphatic vessels relatively difficult. The D2-40 expression of tumour cells in the present study was generally heterogeneous being displayed in 2 patterns: diffuse expression and focal expression (expression at the proliferating periphery of the tumour cell nest with no expression in the central areas) [Table/Fig-7d,e & 8e].
Concurrent with the Zhao D et al., [6] we also observed that the intratumoural lymphatic vessels often had a reticular architecture with numerous tiny or ill defined lumina that differed markedly from the larger and more conventional architecture of vessels found at the tumour margin (peri-tumoural lymphatic vessels) [Table/Fig-7b & c].
In accordance with Yuan P et al., we also noticed high D2-40 expression in basal cell layer of hyperplastic and dysplastic epithelium adjacent to the tumours and in histologically normal squamous epithelium adjacent to squamous cell carcinoma. D2-40 expression was not detected or was extremely low in basal cells of normal epithelium indicating its role in tumour genesis [15][Table/Fig-7f]. Moderate to high D2-40 positivity was also observed in tumour stroma as well as in salivary gland ducts & myoepithelial cells [Table/Fig-7g&h]. Further evaluation of D2-40 expression in potential malignant disorders may explain its involvement in initiation and progression of intraoral cancer originating from potential malignant disorders, which still remains unclear [15].
Comparison of CD34 positive vessels in IT and PT area of OSCC revealed higher MVD in PT area than IT area regardless of the degree of differentiation [Table/Fig-8b,c]. Interestingly, in the present study two patterns of CD34 positive vascular distribution were observed in the cancer nest area, which is in accordance with Nagatsuka H et al., [16]. In the first pattern, designated as circumscribing type, micro vessels encircle the cancer cell nest but do not invade the cancer nest known to represent neovessels with strong remodeling activity. This pattern was recognized in 4 cases [Table/Fig-8d]. In the second pattern, designated as penetrating type, the stroma is narrow, and micro vessels invade the cancer cell nest, known to represent actively proliferating endothelial cells or immature neovessels. This pattern was recognized in 22 cases [Table/Fig-8e].
Conclusion
OSCC exhibits more numerous and dilated LV and MV in PT area, regardless the degree of differentiation. D2-40 can be used as a marker to differentiate between lymphatic vessels and blood vessels. It is not only expressed by lymphatic endothelial cells but also by tumour cells as well as hyperplastic and dysplastic epithelium adjacent to the tumour, indicating its role in tumour progression and suggesting its role in the process of differentiation. Future studies relating D2-40 expression and survival rate of patients with OSCC, and its expression in potential malignant disorder, especially “oral sub-mucous fibrosis” may provide better insight in predicting prognosis and pathogenesis of these lesions which are highly rampant in India. The CD34 exhibits two types of pattern in cancer nests, penetrating type and circumscription type. Previous studies suggested that penetrating pattern of CD34 was associated with a significant risk of metastasis, compared with Circumscribing type. Thus preoperative biopsies followed by additional staining for D2-40 and CD34 may be important prognostic indicators for disease progression and for deciding on therapeutic strategies for patient with oral cancer.
*p<0.05- statistically significant