Periodontitis is defined as inflammation of gingival tissues together with a measurable loss of the attachment of the periodontal ligament and bony support [1]. Different types of bone deformities can result from periodontal disease. Periodontal osseous defects represent a major challenge for the clinician. As compared to horizontal bone loss, cases with angular/intrabony defects have greater chances of bone regeneration if the contour of existing bone and the number of osseous walls are favourable [2].
Amongst various treatment modalities, grafting of biomaterials/bone substitute have been successfully used to accomplish the reconstruction of lost attachment apparatus in deep intra-osseous defects [3]. Bone replacement grafts include autografts, allografts, xenografts, and alloplasts. Alloplastic materials are synthetic, inorganic, biocompatible, or bioactive bone graft substitutes that have been widely used owing to easy availability [4].
Calcium phosphate ceramics have been widely used as bone graft substitute for treating periodontal intrabony defects and have produced clinically significant results [5–7]. Over a five year study, Yukna et al., has found that bone grafts have produced clinically superior results that have remained stable over time [8]. Calcium phosphate bone grafts are available in different forms viz. hydroxyapatite (HA) and tricalcium phosphate (TCP). Both forms fulfill the requirements for synthetic biomaterials being nontoxic, nonantigenic, noncarcinogenic, stable after sterilization and reasonably inexpensive to fabricate [9].
HA biomaterials are complex calcium phosphates which resemble bone mineral in their chemical composition {Ca10(PO4)6(OH)2} and has calcium-to-phosphate ratio of 1.67 [10]. These are available in various forms like HA cement, nonporous HA, porous HA, nano-sized HA and as bioceramics which exhibit a denser structure. HA present biocompatibility with little inflammatory response when implanted within connective and bone tissues. Moreover, deposition of bone mineral crystals may occur directly onto the surface of implanted apatite particles [3].
Tricalcium phosphate (TCP) another class of bone grafting materials has been shown to partially bioresorb when implanted. Tricalcium phosphate has a chemical composition of {Ca3(PO4)2} with a calcium-to-phosphate ratio of 1.5 and is mineralogically β-whitlockite [10]. Highly purified beta-tricalcium phosphate (β-TCP) which is biocompatible and biodegradable has been manufactured. β- TCP degrades during bone remodeling process and is replaced by mature new bone; this material was shown to have good biocompatibility and osteoconductivity in clinical settings[11].
Extensive investigations of HA and β-TCP as bone graft materials in the repair of periodontal defects have been shown to produce more favourable results than other graft materials [5–8,12].
Nano-sized HA (NHA) has been recently introduced as a bone graft material. The first reported case of treatment of human periodontal bone defect with nanocrystalline HA was by Kasaj A et al., [13] who found significant improvement in clinical outcome when compared to open flap debridement. Since limited review regarding the comparison NHA and β-TCP is available, an attempt has been made through this study to compare and evaluate the clinical efficacy of these products as bone graft materials.
So, this study was instituted with the aim to investigate and evaluate clinical and radiological outcomes of regenerative periodontal therapy using NHA and β-TCP in the treatment of human periodontal vertical/angular defects.
Materials and Methods
1. Study Population and experimental design
In this split mouth study, 12 patients (6 males and 6 females) suffering from moderate to severe periodontitis were selected amongst the patients visiting the outpatient Department of Periodontology and Oral Implantology, Guru Nanak Dev Dental College and Research Institute, Sunam, Punjab (India). Criteria for patient inclusion was: (1) Patients in the age group between 20-50 years; (2) Patients who had not undergone any type of regenerative periodontal therapy 6 months prior to the initial examination; (3) Presenting with almost similar periodontal involvement bilaterally (with 2-walled and/or 3-walled defects) as determined by clinical and radiographic assessment with PPD≥5mm; (4) Radiographic presence of bilateral intrabony defect with depth ≥2 mm. Criteria for patient exclusion was: (1) Patients who were pregnant or lactating; (2) Allergic to materials and drugs used or prescribed in this study; (3) Smokers; (4) Teeth with > Millers Grade I mobility [14]; (5) Suffering from any systemic disease or systemic medications that could interfere with post surgical healing process
The study design was approved by the Ethics Committee of Guru Nanak Dev Dental College and Research Institute, Sunam. The nature of this investigation was explained in detail, and the patients signed an informed consent form.
Tweleve patients with a total of 24 periodontal angular/vertical defects which were almost identical as determined clinically and radiographically were selected for the study. After Phase I Therapy, the selected sites were divided into two groups:
Group-I: Periodontal flap surgery was carried out in the upper/ lower right quadrant and synthetic nano-sized hydroxyapatite bone graft (Sybograf®, Eucare pharmaceuticals (P) Ltd., India) was placed in the defect. Sybograf® is a synthetic bio-compatible, bio-resorbable bone graft with a particle size of 20 nm.
Group-II: Periodontal surgery was carried out in the upper/lower left quadrant and β-tricalcium phosphate (RTR®, Septodont, France) placed in the defect. RTR® is a synthetic β-TCP graft with a particle size in the range of 500μm to 1mm with macropore size from 100-400μm and micropore size <10μm.
In both the groups of study, surgery was done on the same day and the following clinical parameters were recorded at baseline, three months and six months postoperatively:
Probing pocket depth (PPD)
Clinical attachment level (CAL)
Gingival recession (GR)
All the measurements were standardized using customized acrylic stents with grooves [Table/Fig-1], which were prepared on the study model of the patients. The recordings were made using a William’s periodontal probe (PWD, Hu-Friedy® Immunity, USA).
Pre-operative probing pocket depth using acrylic stent at baseline
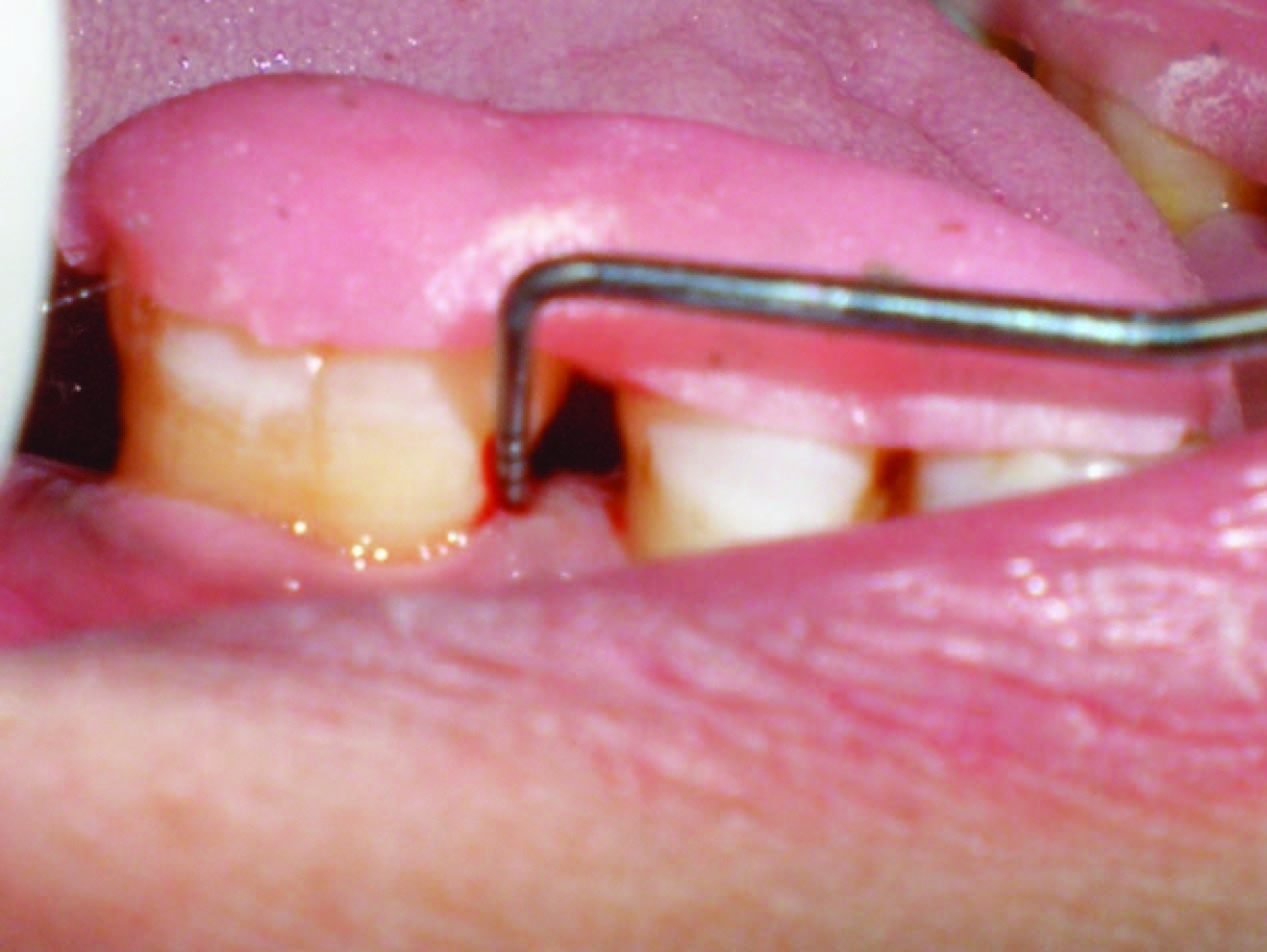
The occlusal stents for vertical probing were made using cold cured pink acrylic and were made to cover the occlusal as well as coronal one-third of the buccal and lingual surfaces of the tooth involved and one tooth mesial and distal to the involved tooth. Vertical grooves were made to guide the probe penetration vertically in the same plane every time it was inserted for recording the measurements. The lower/apical limit of the vertical grooves was used as the fixed reference point (FRP) for the vertical probing depths. If the fixed reference point was between two markings of the probe, then the reading was rounded off to the next highest millimeter. In cases where gingival recession was not evident at baseline measurement, distance from fixed reference point to cemento-enamel junction was measured at the time of surgery.
A. Vertical measurements for determination of probing depth, clinical attachment level and gingival margin position:
Fixed reference point (FRP) to the base of pocket (BOP)
Fixed reference point (FRP) to cementoenamel junction (CEJ)
Fixed reference point (FRP) to gingival margin (GM)
The following calculations were made from the clinical measurements recorded:
Probing pocket depth (PPD):
(FRP to BOP) – (FRP to GM)
Clinical attachment level (CAL):
(FRP to BOP) – (FRP to CEJ)
Gingival Recession (GR):
(FRP to GM) – (FRP to CEJ)
B. Radiographic Measurements
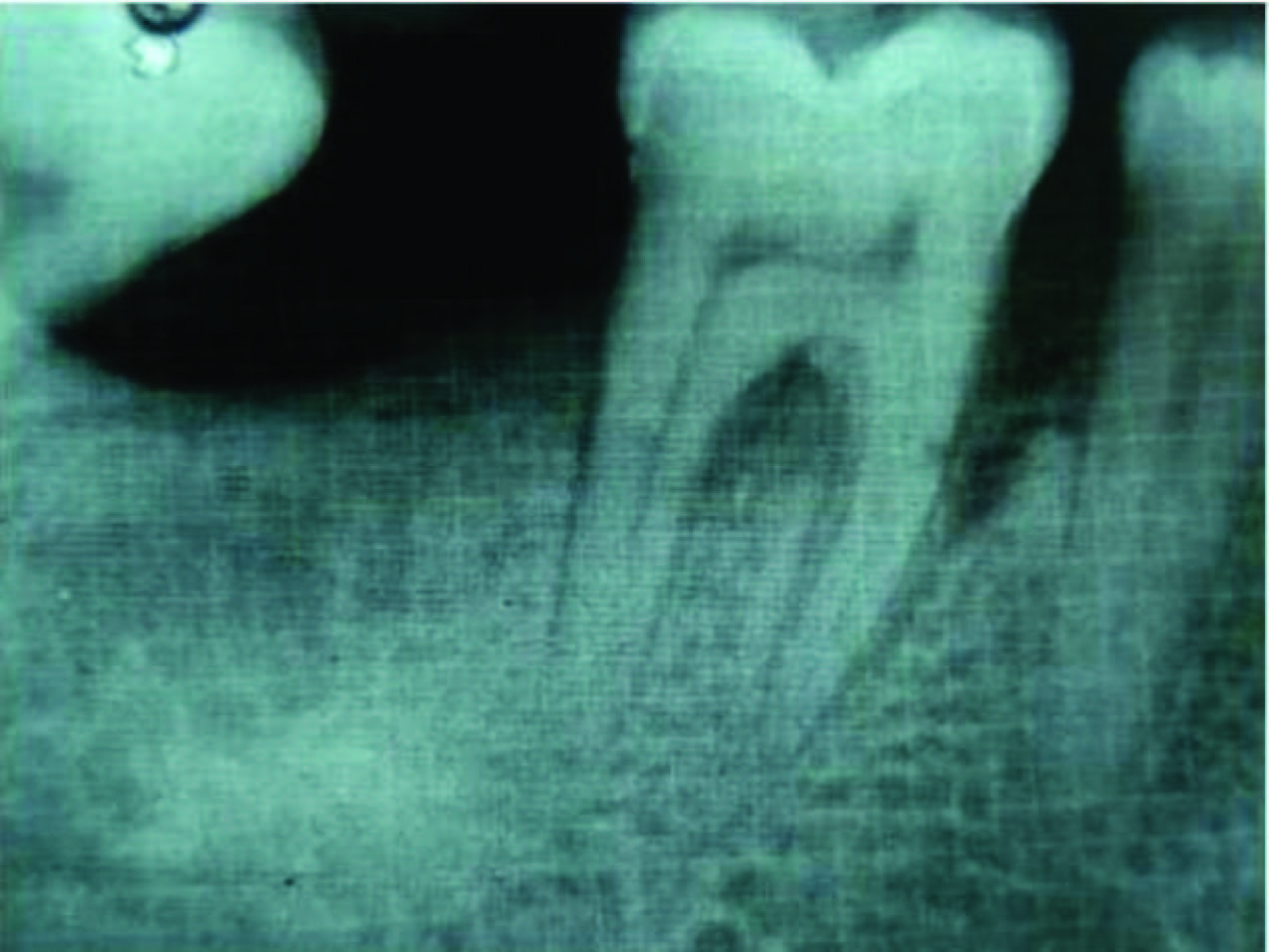
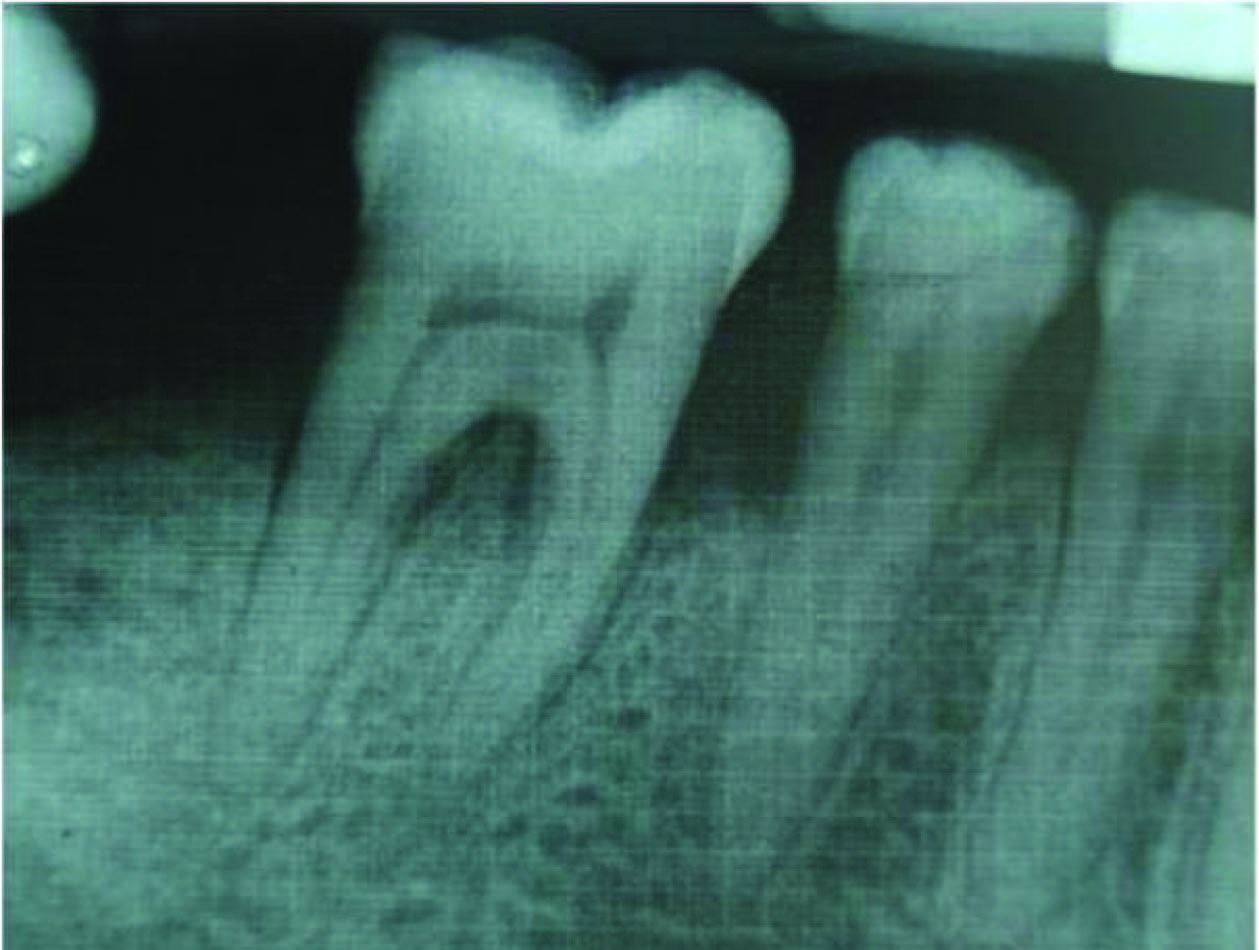
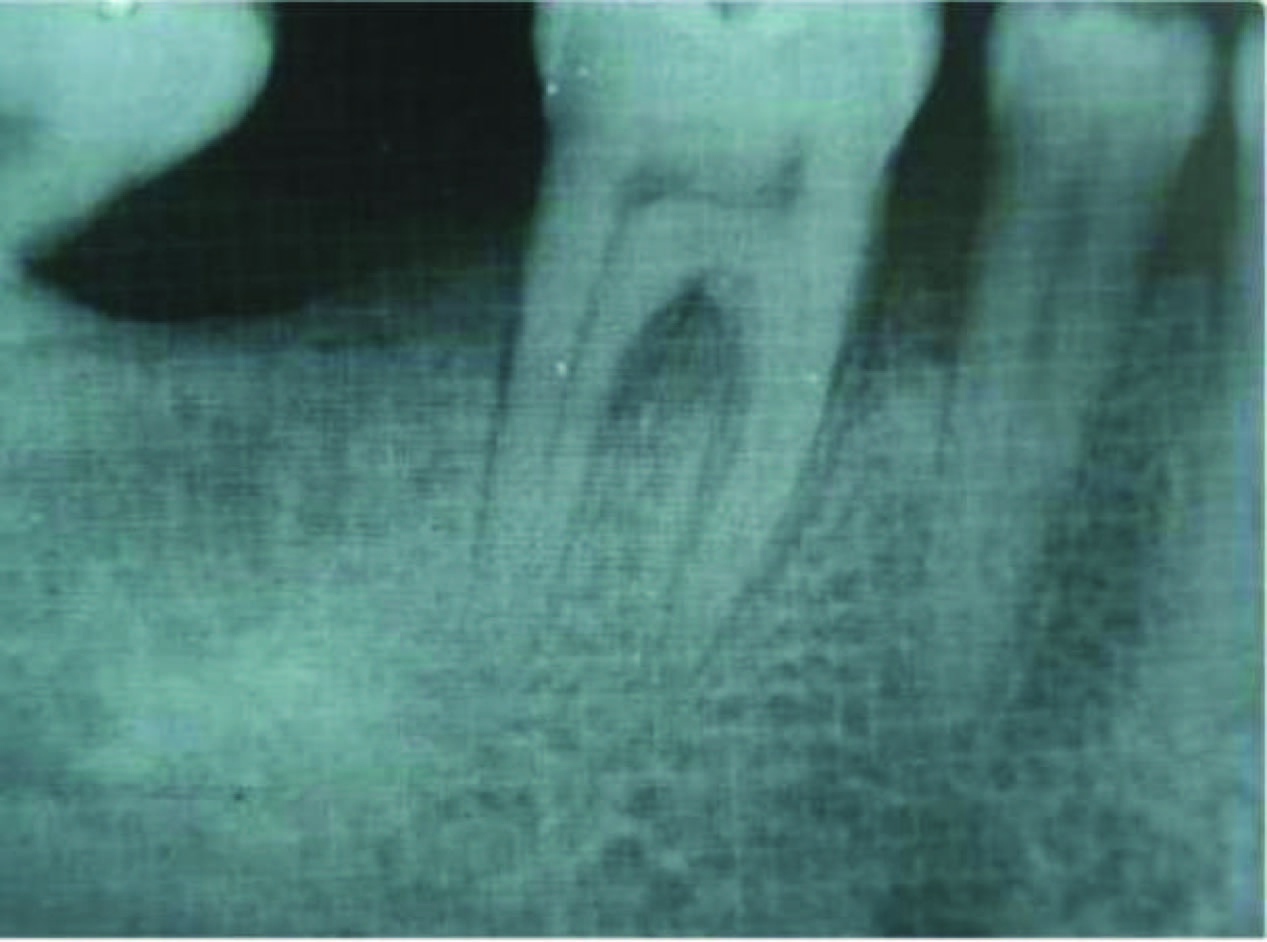
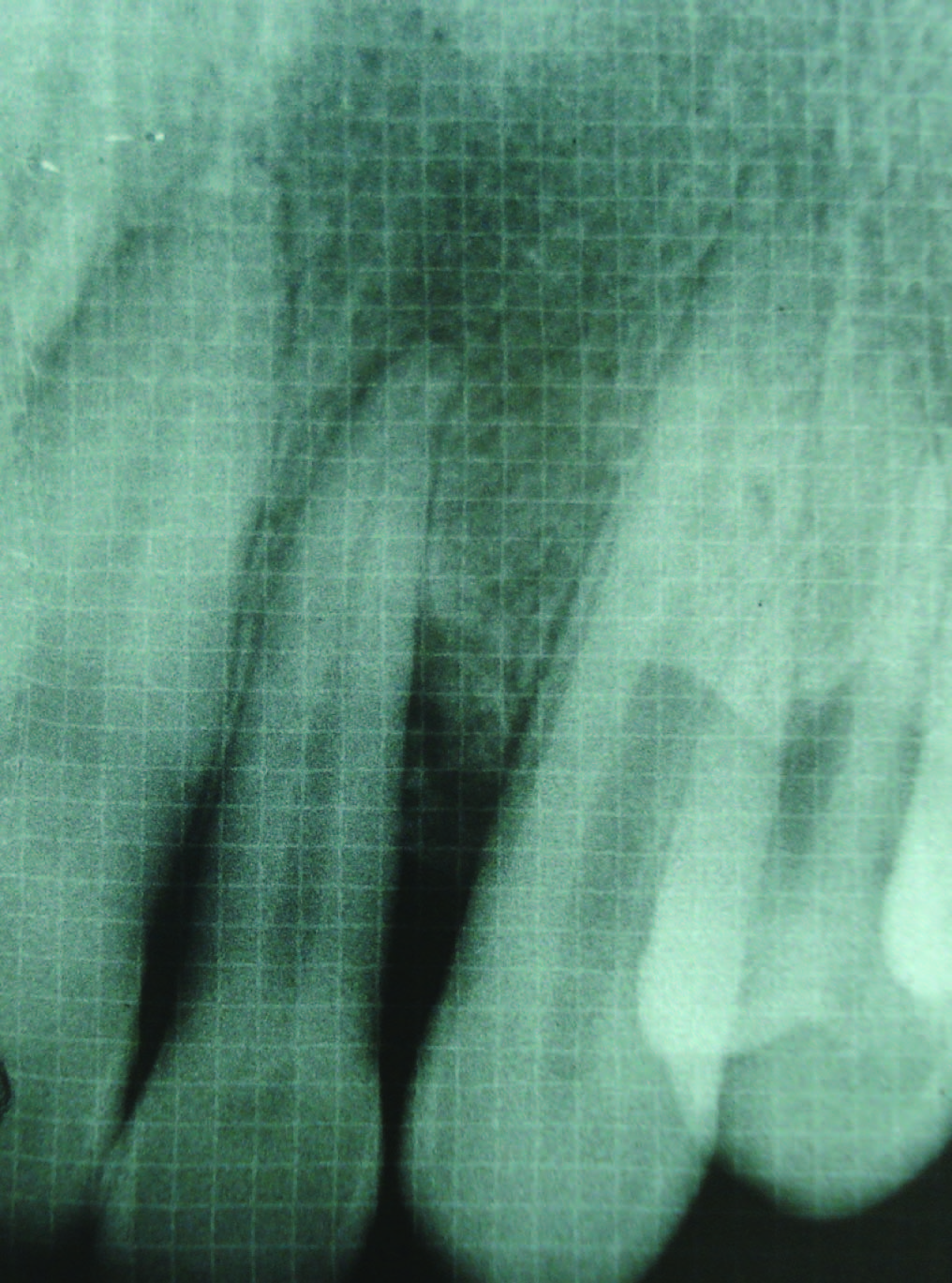
Radiographic defect fill was measured using Intra-oral periapical radiograph and a calibrated grid using long cone/paralleling technique were taken at the baseline [Table/Fig-2,3,4,5], three months and six months postoperatively [Table/Fig-6&7].
Intrabony defect in Group I at baseline
Bone fill after 3 months (Group I)
Bone fill after 6 months (Group I)
Intrabony defect in Group II at baseline
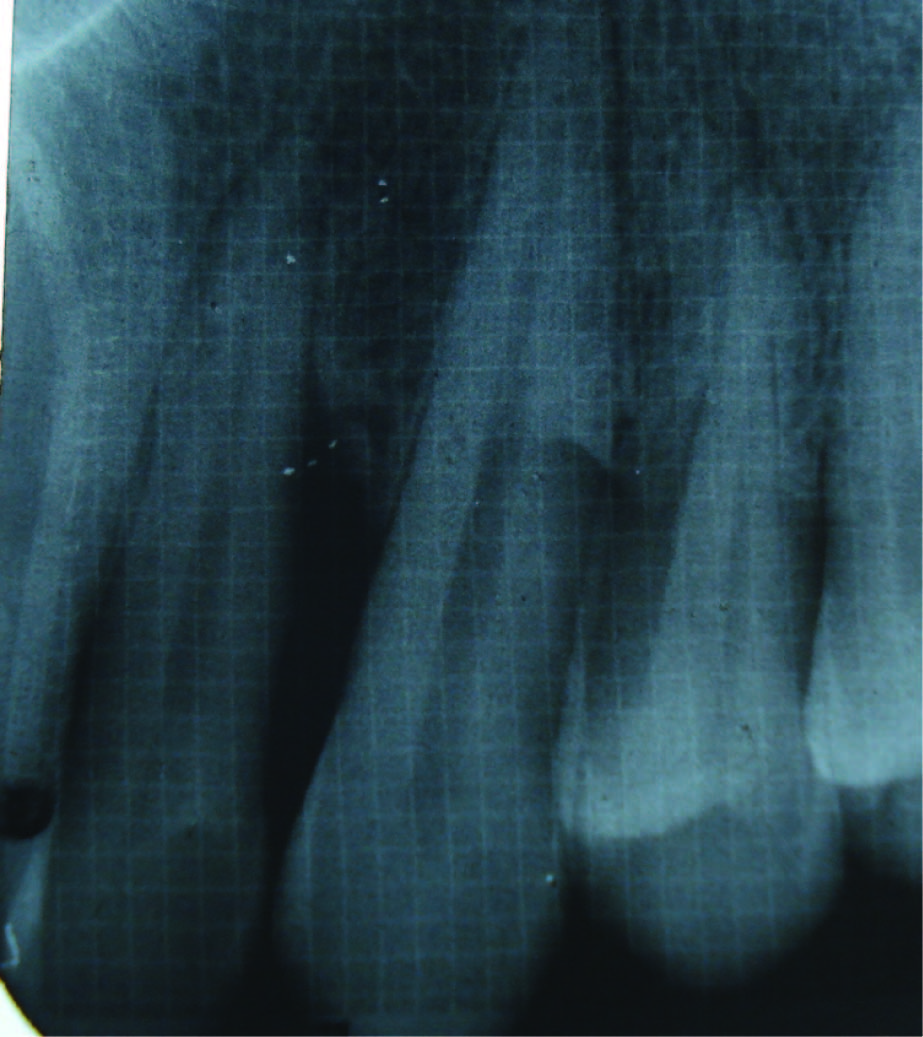
Bone fill after 3 months (Group II)
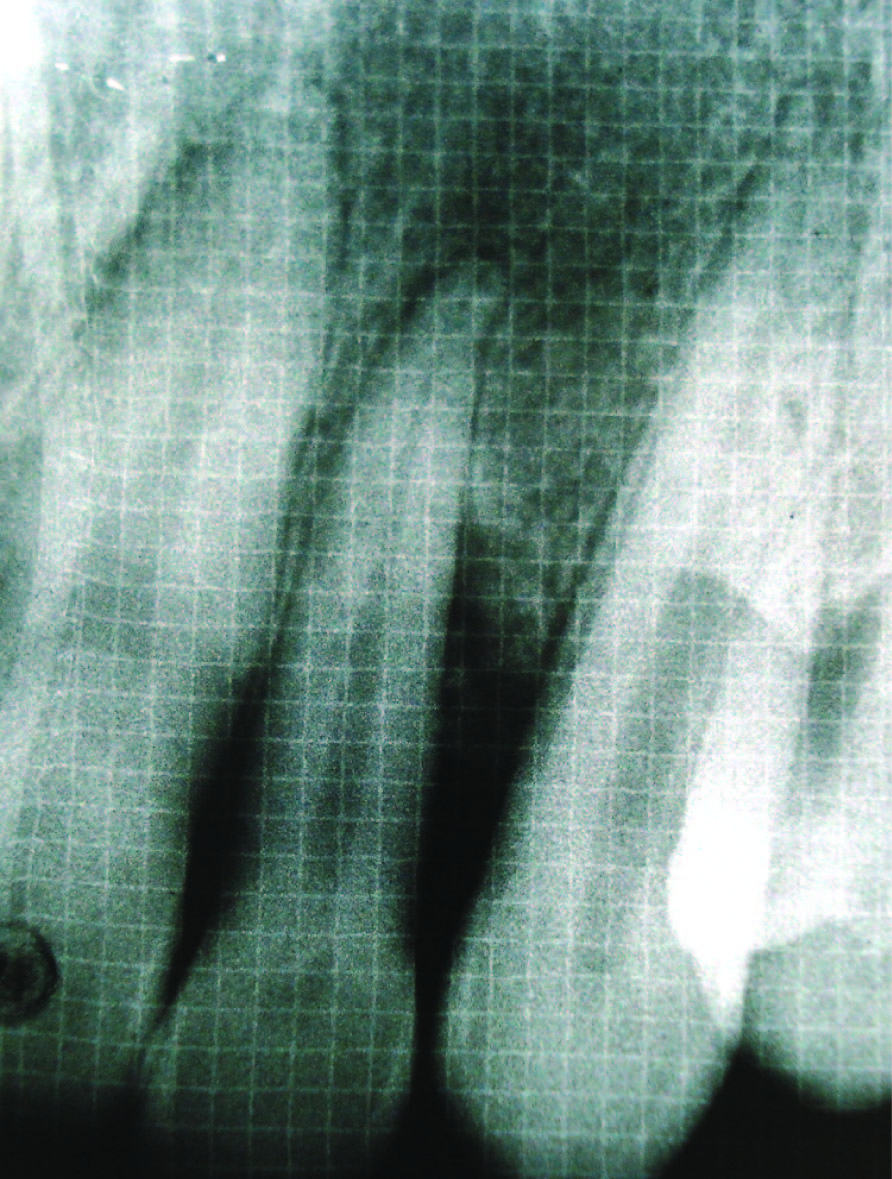
Bone fill after 6 months (Group II)
The radiographs were standardized using Rinn-XCP instrument (Dentsply) and a radiographic grid calibrated in 1x1 mm2. The distance was measured by counting the millimeter markings on the radiograph. The distance from CEJ to the base of the defect was determined by counting the number grid calibrations on the radiograph from the CEJ to the most apical radiolucent point in the defect. The distance from CEJ to the alveolar crest was similarly determined by counting the number of grid calibrations on the radiograph from the CEJ to the most coronal Radiopaque point on the alveolar crest.
The following calculation was made from the radiographic measurements recorded:
1. Radiographic defect fill (RDF):
(Distance from CEJ to the base of the defect) – (Distance from CEJ to alveolar crest).
2. Surgical procedure
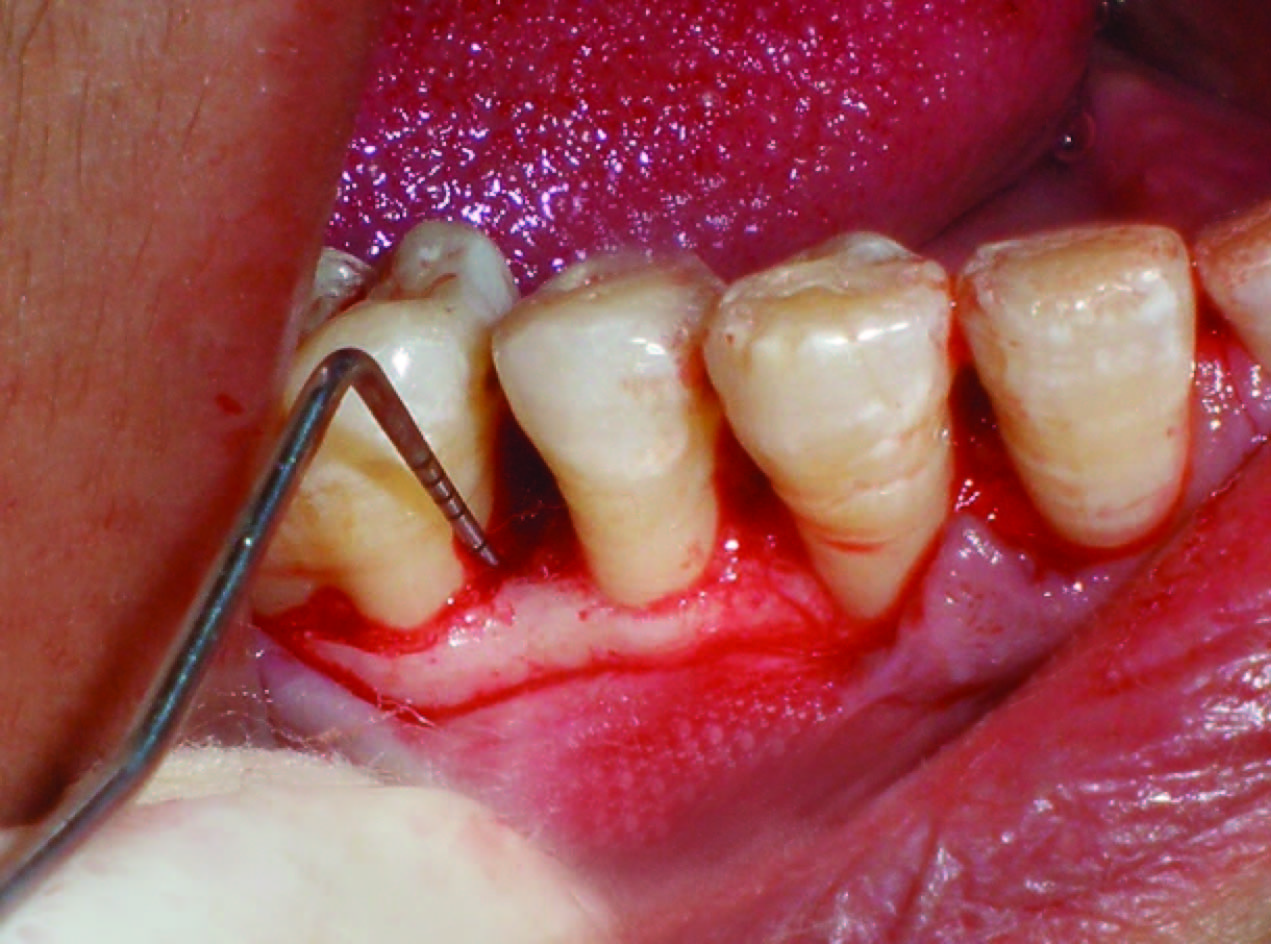
All patients were made to rinse with 0.2% Chlorhexidine digluconate mouthrinse for 30 sec prior to the surgery. Local anesthesia was obtained using 2% lignocaine HCl with adrenaline bitartrate (1:2,00,000) using block and infiltration techniques. The crevicular incisions were given in the localized area, involving one tooth each both mesially and distally along with the involved tooth with the defect on facial and lingual sides using a bard parker knife with blade. A full thickness mucoperiosteal flap was raised to provide access to the intrabony defect and surrounding alveolar bone [Table/Fig-8]. The defect was cleared of granulation tissue and the exposed root surface thoroughly planed to a smooth hard surface. The surgical area was then washed with normal saline and carefully inspected for any remaining granulation tissue or deposits. Any adherent granulation tissue was trimmed from flaps.
Intrabony defect after flap elevation
In the Group I, defects were filled with NHA bone graft material. The required quantity of the graft material was transferred from the vial to the dappen dish and mixed with saline. When it became a cohesive mass, it was delivered in to the vertical defect in small increments [Table/Fig-9]. Carefully each defect was packed adequately to the level of the remaining bony walls of the defect. Similarly in group II, defects were filled with β-TCP bone graft material [Table/Fig-10].
Bone graft placement in Group I (NHA)
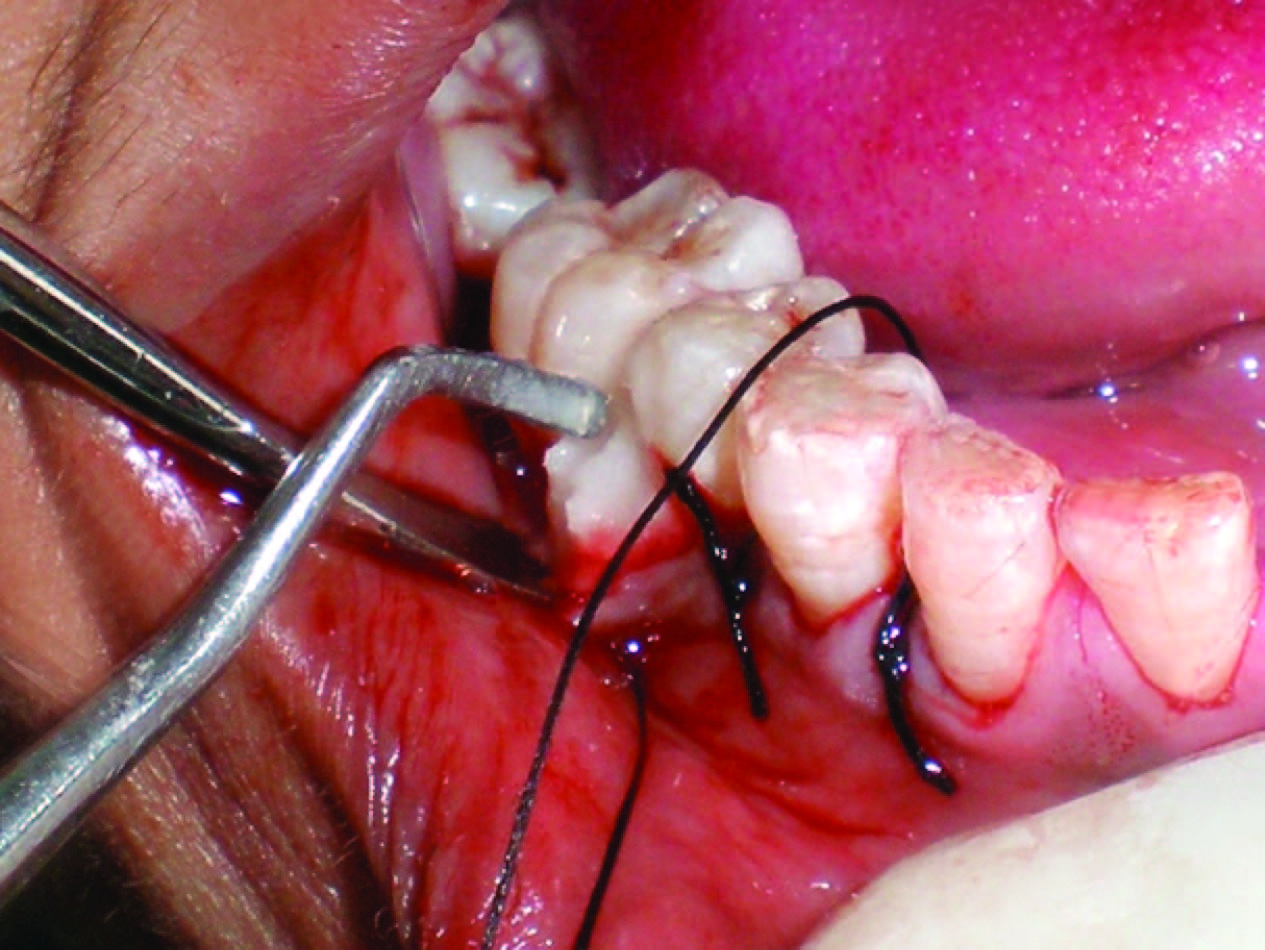
Bone graft placement in Group II (β-TCP)
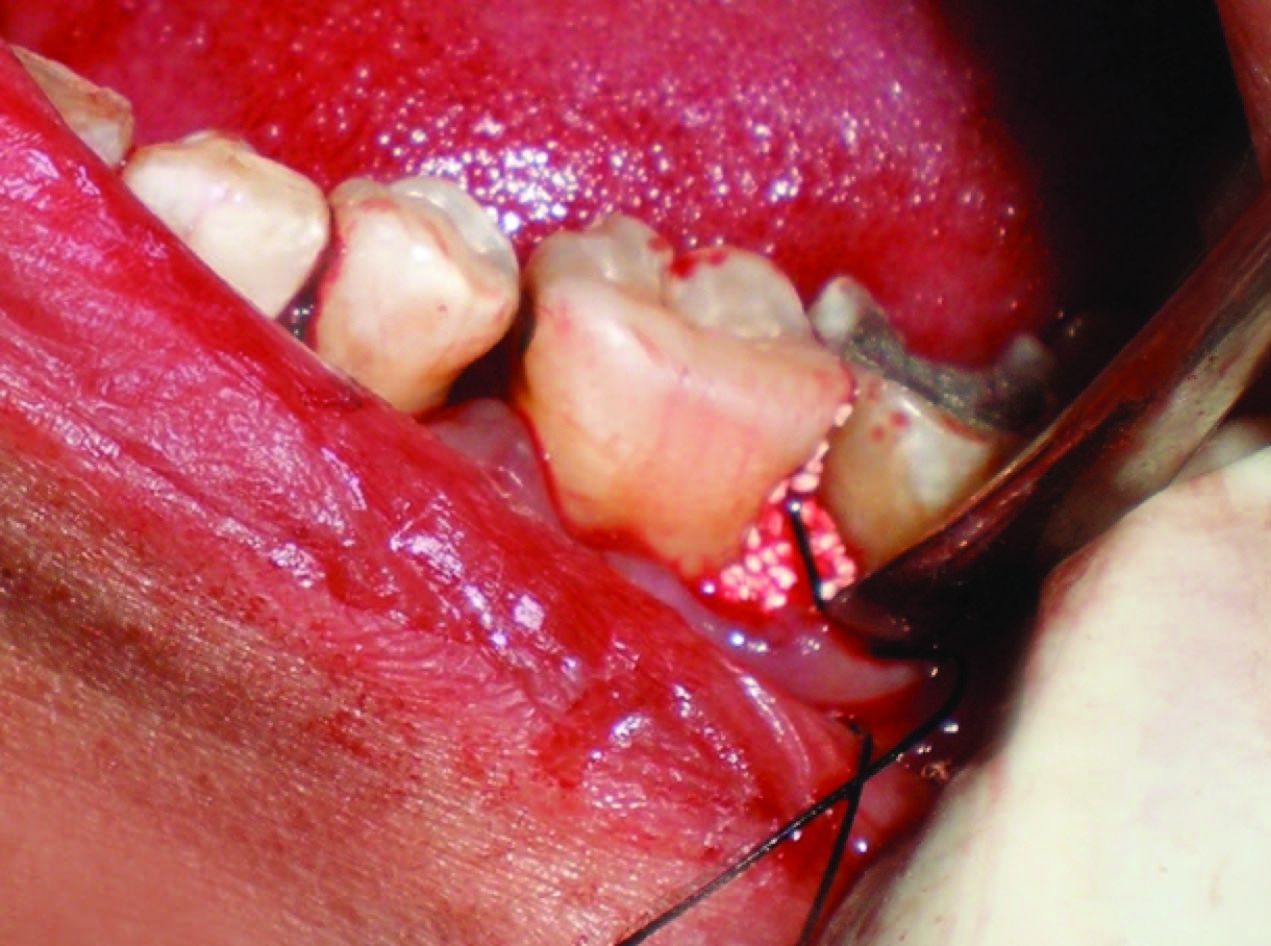
Flaps were then repositioned and secured in place using 3-0 black braided silk and interrupted sutures were placed to obtain primary closure. The surgical areas were protected with a non-eugenol dressing (Coe-PakTM, GC America, IL, USA). All subjects received postoperative instructions and medications, including rinsing with 0.2% chlorhexidine (twice daily for two weeks). Antibiotic (Amoxicillin 500 mg) and anti-inflammatory (ibuprofen 400mg) was prescribed eight hourly for one week. Patients were recalled for removal of sutures on 7th postoperative day.
Results
It was observed that both the materials were well tolerated by all the patients with no adverse reaction, infection or delayed healing during the course of study. Clinical and radiographic measurements were recorded at baseline and at three months and six months postoperatively. The recorded data was put to statistical analysis using Student t-test and the results obtained were compared.
Probing pocket depth (PPD): Statically significant (p<0.001) reduction in Probing pocket depth in both the groups was seen after three months and six months [Table/Fig-11,12]. Intergroup comparison [Table/Fig-13] revealed that greater reduction in pocket depth was recorded in Group I after 3 months with a mean difference of 1.00±0.09mm which was found to be statistically significant (p<0.05). However, after six months, this difference was found to be statistically insignificant. Thus, implying that early pocket reduction was obtained with nano-sized hydroxapatite (Group I).
Clinical attachment level (CAL): Statistically significant (p<0.001) clinical attachment gain was recorded in both the groups after three months and after six months [Table/Fig-11]. The mean CAL gain in Group I after three months and six months was 2.75±0.96 mm and 3.16±0.83mm respectively whereas in Group II mean CAL gain during similar time intervals was 2.58±0.79mm and 3.05±0.73mm [Table/Fig-12]. Intergroup comparison [Table/Fig-13] between two groups showed in–significant (p>0.05) difference in CAL gain at different time intervals. However, greater CAL gain was obtained in Group I after six months.
Gingival Recession (GR): For both the groups, there was significant increase in the mean gingival recession post-operatively. In Group I, the mean GR at baseline was 0.58±0.66 mm which increased to 1.67±0.88 mm after three months while it decreased to 1.58±1.16 mm after six months. Whereas in Group II, there was a constant increase in GR value after three months and six months [Table/Fig-11]. Intergroup comparison [Table/Fig-13] showed insignificant difference in the values of GR at all time intervals.
Radiographic defect fill (RDF): Statistically significant (p<0.001) mean RDF of 1.66±0.61 mm and 0.91±0.57 mm was seen in Group I and II after three months respectively which was further increased to 1.83±0.57 mm and 1.58±0.51 mm after six months [Table/Fig-12]. When compared between the two groups [Table/Fig-13] greater bone fill was found in Group I after three months with a mean difference of 0.75±0.09 mm which was found to be statistically significant (p<0.05). However, after six months insignificant difference in the RDF was found in both the groups (p>0.05). Greater bone fill was found in Group I as compared to Group II after six months with a mean difference of 0.25±0.06 mm (p>0.05)
Mean values of variables (n=12) at different time intervals (Mean ± SD) (In millimeter)
| Variable | Group | Baseline | 3 months | 6 months |
|---|
| Probing Pocket Depth | Group I | 6.83 ± 0.58 | 2.92 ± 0.67 | 2.42 ± 0.67 |
| Group II | 6.42 ± 1.16 | 3.50 ± 0.67 | 2.25 ± 0.96 |
| Clinical Attachment Levels | Group I | 7.17 ± 1.26 | 4.42 ± 1.37 | 4.00 ± 1.04 |
| Group II | 6.67 ± 1.07 | 4.08 ± 0.66 | 4.08 ± 0.79 |
| Gingival recession | Group I | 0.58 ± 0.66 | 1.67 ± 0.88 | 1.58 ± 1.16 |
| Group II | 0.42 ± 0.66 | 1.58 ± 1.08 | 1.83 ± 0.83 |
| Radiographic Defect Depth | Group I | 2.16 ± 1.06 | 0.50 ± 0.45 | 0.33 ±0.65 |
| Group II | 1.92 ± 0.66 | 1.00 ± 0.42 | 0.33 ± 0.42 |
Intra-group comparison showing mean change in values of variables (n=12) at different time intervals (Mean ± SD) (In millimeter)
| Variable | Group | Baseline & 3 months | P value | Baseline & 6 months | P value | 3 months & 6 months | P value |
|---|
| Probing Pocket Depth | I | 3.91±0.99 | <0.001 | 4.41±1.08 | <0.001 | 0.50±0.78 | =0.05 |
| II | 2.91±0.90 | <0.001 | 4.16±0.93 | <0.001 | 1.25±0.75 | <0.001 |
| Clinical Attachment Level | I | 2.75±0.96 | <0.001 | 3.16±0.83 | <0.001 | 0.41±0.51 | <0.05 |
| II | 2.58±0.79 | <0.001 | 3.05±0.73 | <0.001 | 0.67±0.60 | >0.05 |
| Gingival Recession | I | 1.08±0.66 | <0.001 | 1.00± .04 | <0.05 | 0.08±0.51 | >0.05 |
| II | 1.16±0.71 | <0.001 | 1.41±0.51 | <0.001 | 0.25±0.45 | >0.05 |
| Radiographic Defect Fill | I | 1.66±0.61 | <0.001 | 1.83±0.57 | <0.001 | 0.17±0.20 | >0.05 |
| II | 0.91±0.57 | <0.001 | 1.58±0.51 | <0.001 | 0.66±0.49 | <0.05 |
Inter-group comparison showing mean change in values of variables and their mean difference (n=12) at different time intervals (Mean ± SD) (In millimeter)
| Variable | Group | Baseline & 3 months | Mean difference | P | Baseline & 6 months | Mean difference | p | 3months & 6 months | Mean difference | p |
|---|
| Probing Pocket Depth | I | 3.91±0.99 | 1.00±0.09 | <0.05 | 4.41±1.08 | 0.25±0.15 | >0.05 | 0.50±0.78 | 0.75±0.03 | <0.05 |
| II | 2.91±0.90 | 4.16±0.93 | 1.25± |
| Clinical Attachment Level | I | 2.75±0.96 | 0.17±0.17 | >0.05 | 3.16±0.83 | 0.11±0.10 | >0.05 | 0.41±0.51 | 0.06±0.09 | >0.05 |
| II | 2.58±0.79 | 3.05±0.73 | 0.35±0.60 |
| Gingival Recession | I | 1.08±0.66 | 0.08±0.05 | >0.05 | 1.00±1.04 | 0.41±0.53 | >0.05 | 0.08±0.51 | 0.17±0.06 | >0.05 |
| II | 1.16±0.71 | 1.41±0.51 | 0.25±0.45 |
| Radiographic Defect Fill | I | 1.66±0.61 | 0.75±0.09 | <0.05 | 1.83±0.57 | 0.25±0.06 | >0.05 | 0.17±0.20 | 0.49±0.29 | >0.05 |
| II | 0.91±0.57 | 1.58±0.51 | 0.66±0.49 |
Discussion
Broader understanding of the pathogenesis of periodontal diseases and of tissue reactions to surgical procedures have led to considerable progress in the periodontal therapy. In particular, new treatments and adjuncts to existing therapeutic modalities are constantly being developed and introduced in the field of periodontal regeneration [15].
The treatment modalities aimed at periodontal regeneration include root conditioning, use of growth factors, barrier membranes (GTR), bone grafting or a combination of these. Ample histologic evidence is available regarding osseous grafts, in humans, demonstrating reconstruction of periodontal unit which comprised of new cementum, alveolar bone and a functional periodontal ligament [16]. Since the advent of nanotechnology, various materials have been introduced for the treatment of the bone defects which have shown promising results.
This study evaluated the clinical effectiveness of two Calcium phosphate ceramic bone grafts in the treatment of periodontal defects. Both treatment modalities produced statistically significant and clinical improvement in PPD, CAL and RDF.
The mean reduction in PPD of Group I (NPH) and Group II (β-TCP) after three months was 3.91 ± 0.99 mm and 2.91 ± 0.90 mm and after six months was 4.41± 1.08 mm and 4.16 ± 0.93 respectively which means that early reduction in probing depth was seen in Group I. Similar trends were observed for CAL gain in both the groups. The comparative assessment of the two groups for gain in CAL showed that the mean value of gain in clinical attachment levels for Group I at different time intervals was higher than Group II. However, these differences in CAL were not statistically significant.
The comparative evaluation of the two groups for mean radiographic defect fill was found to be higher in Group I after three months post-surgery with mean difference of 0.75 ± 0.09 mm. This difference was found to be statistically significant at 5% level of significance. Greater defect resolution was seen in Group I as compared to Group II at six months postsurgery.
Decrease in probing depth, gain in clinical attachment level [17] and a substantial amount of bone fill [8,18] has been previously reported in hydroxyapatite based biomaterials. Although reduction in probing depth may not reflect regeneration, gain in CAL and radiographic defect fill provides a good support of evidence for resolution of intrabony defects. HA biomaterials are generally non-bioresorbable over periods of many years. In healed defects, HA appears to act as a mild foreign body, usually surrounded by dense connective tissue [19].
A special feature of nanostructured materials is an extremely high number of molecules on the surface of the material. Schnettler et al., [20] found that NHA binds to the bone and stimulates bone healing by stimulation of osteoblast activity. Early resorption in nanoparticle HA (12 weeks) has been reported by Thorwarth M et al., [12] which may explain the fact that greater bone formation was observed in Group I at 3 months which may have lead to early radiographic changes. Strietzel FP et al., [21] have found that nanocrystalline HA material to be different from microcrystalline bone substitution materials regarding solubility. Its chemical composition corresponds to that of bone mineral and the particle size helps in accelerated substitution by vital bone.
β-TCP is a resorbable bone graft [22]. Trisi P et al., [23] reported β-TCP has a phase purity of 99%, which makes it completely resorbable simultaneous with new vital bone formation within a period of 6-12 months. This explains the fact that greater bone formation was seen in Group II at 6 months post-operatively than at 3 months. Tricalcium phosphate ceramic serves as a nidus for new bone formation in the intrabony defect as well as in the close environment. The granules are embedded in a dense fibrous framework with minimal inflammation. In animal experiments, β-TCP was found to gradually degrade during the bone remodeling process and replaced by mature bone [22].
They resorb and release some of the calcium ions in the biologic fluid that evoke osteogenesis in a bone-tooth environment [24]. The resorption process seems to be mediated by two mechanisms: the first, a disaggregation process and second a removal process [23]. β-TCP may be hydrolysed either partially or completely to hydroxyapatite [11]. The disaggregation process may be coupled with new bone formation. The process of resorption and replacement in open environment may occur over a period of years [22].
The early bone formation in Group I might have resisted deeper probe penetration which would have lead to improvement in clinical parameters including greater PPD reduction and CAL gain which is additionally supported by radiographic defect fill at three months.
The results of our study are in agreement with the study of Evans GH et al., [25] who found no significant difference regarding hard and soft tissue changes except for a significantly lower percentage fill withβ-TCP compared to HA. The histological studies of Froum and Stahl [17] found that there was bone formation at three months in human subjects while others [26–28] observed new cementum formation and new attachment formation, following the placement of HA or β-TCP in periodontal defects. Furthermore, Saffar JL et al., [7] and Ettel RG et al., [29] have reported the osteogenic potential of above mentioned graft materials.
Periodontal reconstructive surgery for intabony defects is a technique sensitive procedure [30]. Clinically, several factors including patient selection, defect morphology, biological and physicochemical characteristics of grafted biomaterials as well as surgical variables and post operative maintenance may alter the extent of clinical attachment gain and bone re-growth following a grafting procedure.
Conclusion
Within limits of the study, both NHA and β-TCP have proved to be beneficial in the management of intrabony defects. Greater pocket reduction, gain in clinical attachment level and defect fill was seen at three months for NHA. Also, slightly greater CAL gains and radiographic defect fill was seen in NHA group after six months. But the comparison between the two groups was statistically in-significant for any of the parameter measured after six months indicating equal efficacy of both materials.
The results from this clinical trial encourage the use of nanosized hydroxyapatite for the early resolution for the human periodontal defects. It is important to emphasize that the data generated by this study is derived from six months observation only. Hence, long term studies with greater sample size are suggested to evaluate the efficacy of the above materials in the treatment of human periodontal intrabony defects.