Introduction: Furcation perforation can have a negative impact on the prognosis of the affected tooth by compromising the attached apparatus. Hence these perforations require immediate repair. A variety of materials have been suggested for repair, of that MTA is the most promising material. The purpose of this study was to compare the ability of Gray and White MTA to seal furcation perforations using a dye extraction method under spectrophotometer.
Materials and Methods: A total of 60 permanent mandibular molars were randomly divided into four experimental groups of 15 samples each as follows: Group A: Perforation repaired with White MTA. Group B: Perforation repaired with Gray MTA. Group C: Perforation left unsealed (positive). Group D: without perforation (negative). Dye extraction was performed using full concentration nitric acid. Dye absorbance was measured at 550 nm using spectrophotometer. The data analyzed using one-way-Anova Ratio and Unpaired t-test showing statistically significance difference among the groups.
Result: It was seen that Group D samples without perforation showed least absorbance followed by Group A (perforation repaired with White MTA) and Group B (perforation repaired with Gray MTA). Group C (perforation left unsealed) showed highest absorbance.
Conclusion: The White and Gray Mineral Trioxide Aggregate performed similarly as a furcation perforation repair material. There was no significant difference between the Gray MTA and White MTA.
Introduction
In endodontic practice, procedural accidents such as furcal perforation may occur and affect the prognosis of root canal treatment. In an analytical study of endodontic failures, Ingle reported that perforations were the second greatest cause of endodontic failure and account for 9.6% of all unsuccessful cases [1].
American association of endodontists (AAEs) Glossary of endodontic terms defines perforation as a mechanical or pathologic communication between the root canal system and the external tooth surface [2].
The perforations can occur due to caries, resorption, or iatrogenic factors like during access cavities, coronal shaping and post space preparation [3]. Regardless of the cause, furcation perforation is followed by bacterial contamination, periodontal fiber destruction, periradicular tissue injury, inflammation, bone resorption [2]. Sinai found that the prognosis for a tooth with a perforation depends on the location of the perforation, time for which the perforation is open to contamination, the possibility of sealing the perforation, and accessibility of the main canal. He stated that middle third and apically situated perforations were less serious than those that occurred in the coronal third of the canal, including furcal perforations [4,5].
Ideally, Samuel Seltzer, Irving Sinai and David August reported that to prevent bacterial contamination, perforations should be repaired as quickly as possible with biocompatible material [6]. In a review article, Alhadain discussed the ideal properties of a perforation repair material. It should be non-toxic, noncorrosive, nonstaining, have bactericidal or bacteriostatic properties, easy to manipulate, set rapidly, well tolerated by periradicular tissues, promote regeneration of periradicular tissues, dimensionally stable, radiopaque, and remain unaffected by the presence of moisture or low pH levels [7].
Numerous materials have been recommended for the repair of this iatrogenic accident including amalgam, calcium hydroxide, tricalcium phosphate, cavit, zinc oxide, hydroxyapatite, glass ionomer, super- EBA cement, composite bonded restorations and decalcified freeze-dried bone [1,5]. However, none of these materials has been able to predictably re-establish the normal architecture in perforated furcation.
In 1990s, a new class of restorative material Mineral trioxide aggregate (MTA) was developed at Loma Linda University as a root-end filling material [8]. Mineral trioxide aggregate is a mechanical mixture of three powder ingredients: 75% Portland cement, 20% bismuth oxide and 5% gypsum [9]. It also contains trace amounts of SiO2, CaO, MgO, K2SO4, and Na2 SO4.
There are two types of MTA available: Gray and White. The principle components of the Gray-coloured formula are calcium oxide, dicalcium silicate, tricalcium silicate, tricalcium aluminate, tetracalcium aluminoferrite and calcium sulphate dihydrate. The more aesthetic White coloured preparation lacks the tetracalcium aluminoferrite. The difference between White and Gray is primarily a result of the lower iron oxide content used in the White MTA. Reasonably, the absence of significant FeO in White MTA causes the colour change from Gray to White, and changes the percentage of silica and alumina in each material. Gray MTA is not used where aesthetic is the prime concern [10]. In cases like lateral perforation or coronal perforation in anterior teeth where aesthetic is more important White MTA is used. Gray MTA gives a discolouration to tooth and gingiva also [11].
Currently, Mineral trioxide aggregate is also being used in other clinical procedures such as apexification, perforation like lateral or furcation, in pulp capping with reversible pulpitis, as a radicular reabsorption, retro obturation and pulpotomy. MTA has a good adherence capacity in the dentine walls, becoming by this reason resistant to dislocation forces. So that it may also be indicated to furcation perforations [12].
In experiment studies have compared the sealing ability and biocompatibility of MTA with those of amalgam, Super EBA, and IRM. The sealing ability of MTA has been shown in dye and bacterial leakage studies to be superior to that of amalgam and to be equal or better than Super –EBA [7].
Hence, this in vitro study was undertaken to evaluate the ability of Gray and White MTA to seal furcation perforations in mandibular molars using a dye extraction leakage method.
Materials and Methods
Sixty human permanent lower molars were collected and used in this study. Collected teeth included lower molar with separate mesial and distal roots (no fused roots), minimal or no caries, no restoration and fracture lines. All teeth were stored in physiologic saline till the time of use. All molars were decoronated 3mm above the cementoenamel junction and roots were amputated 3mm below the furcation area with diamond disc. A standardized endodontic access cavity was prepared in each sample by using Endo access bur followed by Endo Z for lateral extension and finishing of cavity walls [Table/Fig-1]. Pulp tissues and debris were removed and the prepared cavity irrigated with sterile saline. The samples were dried and sticky wax was placed over the orifices of each canal up to the root. Each sample was covered completely including cavity walls and pulpal floor with two successive layers of nail varnish and allowed to dry at room temperature.
The perforation was made with # 4 high speeds round carbide bur while the tooth was hand held. The defect was made from the external surface to ensure that each perforation was centred between the roots. The chamber and perforation were flushed with water and dried. ([Table/Fig-2] perforation from access cavity) ([Table/Fig-3] perforation from root).
Perforation from access cavity
Perforation from root side
Teeth were randomly divided in to four experimental groups of 15 samples each as follows: Group A: Repaired with White MTA. Group B: Repaired with Gray MTA. Group C: Perforation was left unsealed (positive control). Group D: Without perforation (negative control)
Group A & B: All samples were placed in Petri dish containing moistened cotton in an attempt to simulate clinical conditions. Internal matrix, Collacote (Zimmer/dental) was cut with scissor into 2×2 mm pieces. Collacote was packed into the perforation to provide a matrix for packing MTA [Table/Fig-4]. The area apical to the perforation was filled with Collacote by using finger plugger to provide an intimate matrix against which to pack MTA[Table/Fig-5]. White MTA and Gray MTA (Angelus) were mixed according to the manufacturer instructions. The cement was placed on the perforation site in increments using a micro apical placement system (MAP) and condensed with light pressure using finger plugger. Moist cotton pellets were placed over the repair material to allow the MTA to set.
Collacote placed in the perforation
MTA Placed in perforation
Group C: Perforations were left unsealed.
Group D: No perforation was performed.
The samples were kept in 100% humidity at 37oC for 24 h. Methylene blue dye was applied inside the access cavity of each sample. Samples were stored at room temperature for 48 h. Molars were placed under running tap water for 30 min to remove all residues of methylene blue. All samples were then dried and nail paint was removed with a parker blade #15. The samples were placed in vials containing 1 ml of concentrated (65wt %) nitric acid for three days. Vials were centrifuged at 14,000 rpm for 5 min. 1000μl of the supernatant from each vial was transferred to a 96-well plate. Absorbance was read by UV/Vis spectrophotometer UV100 at 550nm using concentrated nitric acid as a blank.
Results
On comparing Mean [Table/Fig-6] and SD values as shown that White MTA - Angelus = 0.127% ±0.0035, Gray MTA - angelus = 0.125% ±0.0041, positive control = 0.820% ±0.1460 and negative control = 0.014% ±0.0106 It was seen that group D showed least absorbance followed by Group A, Group B and Group C showed highest absorbance.
Group A: Perforation Repaired with White MTA; Group B: Perforation Repaired with Gray MTA; Group C: Perforation Left Unsealed (Positive Control); Group D: without Perforetion (Negetive Control)
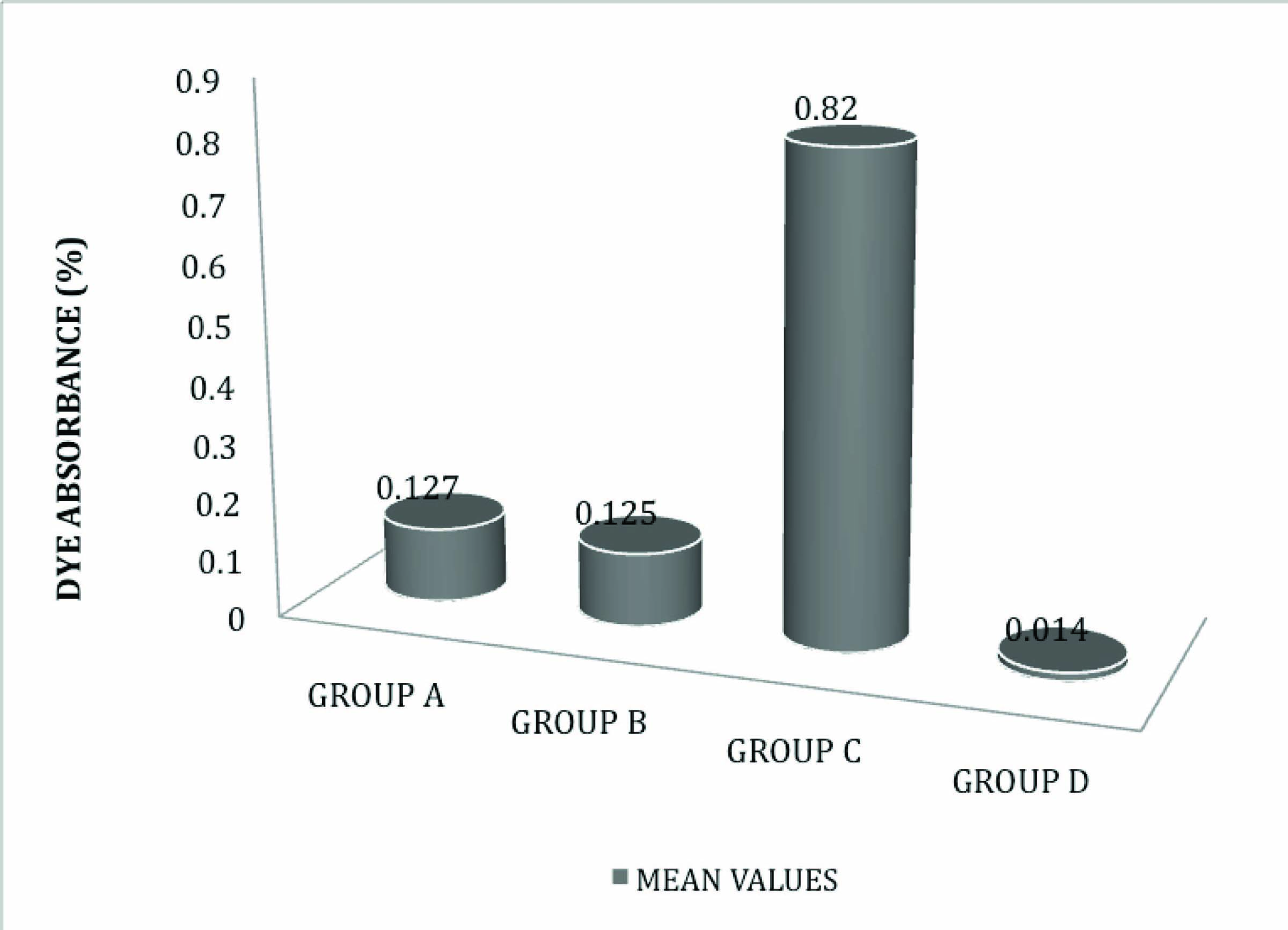
Results showed no statistically significant difference in dye absorbance between the Gray MTA and White MTA (Angelus) (i.e. p<.05).
Discussion
Ingle and Bakland reported that the outcome of endodontic treatment can be adversely affected by procedural accidents such as furcal perforation which affect the prognosis of root canal treatment [1].
Various materials have been recommended for the repair of perforations including amalgam, indium foil, calcium hydroxide, tricalcium phosphate, cavit, zinc oxide and eugenol, hydroxyapatite, glass ionomer, super- EBA cement, decalcified freeze-dried bone, etc. However, the most popular material today is mineral trioxide aggregate (MTA) [7,12,13].
MTA has become very popular as a perforation repair material due to its excellent biocompatibility. It is evidenced to have a favourable biologic response, minimal toxicity and pulpal irritation, mild periapical inflammation, nonmutagenicity, cell adherence and growth, increased levels of alkaline phosphatase and osteocalcin, interleukine production (IL-6, IL-8), periodontal ligament attachment, cementum growth, and promote dentinal bridge formation. One of the properties which make MTA as a material of choice is its ability to set in presence of moisture [9]. Although freshly mixed MTA has a more toxic effect than the set ones, it has been shown that there is no difference in the quantity of cementum or osseous healing associated with freshly placed or set MTA when used as root end filling material [14].
MTA has been shown to leak less than amalgam and Super EBA when used as a perforation repair material, and has been shown to be less cytotoxic and neurotoxic than other repair materials [15,16]. Bonded resins have their intimate adherence to dentin, but may not perform well when used to repair perforation because of their exposure to moisture through the perforation site. Additionally, some of these materials may be cytotoxic [17].
Despite its advantages, MTA may be inconvenient to use because the manufacturer’s directions require that it be covered by a wet cotton pellet and left for at least 3 to 4 h to set. Thus, repair of a perforation discovered or produced during endodontic therapy requires the termination of the procedure and reappointment of the patient after the material has hardened, which is inexpedient for both the patient and the practitioner. This difficulty can be overcome by another commercial form of MTA Angelus (Angelus Solucoes odontologicas, Londrina, Brazil) which has used in this study [4].
However, there are fewer studies evaluating and comparing the sealing ability of Gray and White Pro root MTA. Hence, the present study was under taken to evaluate the difference in the sealing ability of the two types of Gray and White MTA Angelus.
Sticky wax was placed over the orifice of each canal up to the root. Each molar was covered completely including cavity walls and pulpal floor with two successive layers of nail varnish and allowed to dry at room temperature. This was done to ensure that dye could not be absorbed by any other surface of the tooth. To ensure each perforation was centred between the roots, furcation perforations were induced by a # 4 long shank carbide round bur from external surface. This resulted in perforations of almost 2 mm in diameter.
Internal matrix has been advocated to limit the over extension of the repair material. The collagen matrix used in this study is a soft material, which expands because of moisture and has a hemostatic effect. It seems suitable to be used with MTA because this material is a paste and does not require forces of condensation. C Bargholz attempted perforation repairs with mineral trioxide aggregate using a modified matrix concept. In contrast to other treatment concepts, this technique uses collagen as a completely resorbable barrier material and MTA for sealing of the perforation. This not only results in repair of the defect, but also promotes healing of the periodontal ligament as shown by the cases [3].
In this study, White and Gray Mineral Trioxide Aggregate - Angelus was used as a perforation repair material. It was available in powder and liquid. It was mixed according to manufacturer’s recommendations and is used in a slurry form, which gradually hardens in the oral environment. Although the methods for mixing and placing the cement were well standardized in this study, there is evidence that increased water to powder mixing ratio could account for increased solubility and porosity of the material. Because of the handling properties of MTA, it is likely that clinicians vary in the way they mix and place the cement. It is advised that manufacturers’ recommendations for mixing be strictly followed to avoid decreasing the optimum properties of the material.
Moist cotton pellets were placed over the repair material to allow the MTA to set. Molars were kept in 100% humidity at 37oC for 24 h. Methylene blue dye was applied inside the access cavity of all samples and all the samples were kept for 48 h to allow for adequate dye absorbance. Subsequently, all the samples were subjected to dye extraction procedure.
Several methods to evaluate leakage of perforation repair material have been used earlier including dye penetration, bacterial penetration, and fluid filtration methods. The dye penetration technique has long been used in endodontics because of its ease of performance and the fact that it does not require sophisticated materials. However, it had several drawbacks - (a) Molecular size of the dye molecules is smaller than bacteria, (b) It does not measure the actual volume absorbed by sample but merely measures the deepest point reached by the dye. (c) It relies on randomly cutting the roots into two pieces, without any clue of the position of the deepest dye penetration [3]. Torabinejad et al., stated that a material that is able to prevent the penetration of small molecules should be able to prevent larger substances like bacteria and their by-products [12]. Based on this, the dye extraction method seems to be a reliable technique. It takes into account all absorbed dye by the samples [3].
This study followed an established protocol for dye extraction. Methylene blue was placed in nitric acid and evaluated with the spectrophotometer. Maximum optical density was read. Wu MK et al., found that methylene blue was decoloured by MTA obtained from Loma Linda University. They concluded the calcium oxide contained in MTA may react with water, form Ca(OH)2 that decolours methylene blue, and that dye may be further diluted with cooling water used in sectioning teeth for a linear dye penetration study [3].
Negative control samples had low dye absorbance (0.014%, ±0.010) close to that of blank nitric acid, which showed absorbance of 0.010%. This small difference can be attributed to the yellowish colour of teeth, where as blank is colourless. Positive control samples in which perforation was not repaired had the highest dye absorbance of all groups denoting the accuracy of the technique. WMTA (Angelus) had dye absorbance (0.127%, ±0.0035) and GMTA (Angelus) had dye absorbance (0.125%, ±0.0041). There was no statistical significant difference between WMTA and GMTA.
The setting of MTA takes place in two stages. After mixing with water the hydration reaction of both silicates begins and results in the formation of a gel consisting of calcium silicate hydrates, which liberates calcium hydroxide. The calcium hydroxide then gradually reacts with the minerals form other hydrated compounds. The calcium silicates contribute most to the binding power and strength of the material. It is also the main binding agent of crystalline calcium hydroxide that leaches most readily from the gel. Tricalcium aluminate exhibits flash setting on hydration. Tetracalcium aluminoferrite reacts at a slower rate than tricalcium aluminate [18].
Reports available regarding sealing ability, biocompatibility and tissue regenerating ability of MTA, for examples, MTA- dentin interface and MTA marginal adaptation have been studied. Takashi Komabayashi et al., showed that the geometry of small MTA particles (size- 1.5μm) made it possible to enter into open dentin tubules (2-5μm) in regards to size and shape. This might be an important mechanism to provide a hydraulic seal. GMTA powder has particle sizes ranging from 1-10 μm and for WMTA has less than 1 to approximately 30 μm before hydration. WMTA particles are more homogeneous and finer than GMTA particles. Smaller particle size increases the surface available for hydration and causes greater strength as well as ease of handling [19].
The results of this study have been in accordance with studies carried out by Douglas M Ferris et al., [5], Khalid Al-Hezaimi et al., [20], Hatim A. Hamad et al., [3], etc.
Conclusion
Under the limitations of this study, it can conclude that both the White and Gray Mineral Trioxide Aggregate performed a good similar sealing ability as a furcation perforation repair material. There was no significant difference between the Gray MTA and White MTA. To collaborate the results of this study, further studies are required.
[1]. Ford TRP, Torabinejad M, Mckendry DJ, Hong CU, Kariyawasam SP, Use of mineral trioxide aggregate for repair of furcal perforationsOral Surg Oral Med Oral Pathol Oral Radiol Endod 1995 79:756-62. [Google Scholar]
[2]. Hamad HA, Tordik PA, McClanaban SB, Furcation perforation repair comparing gray and white MTA: a dye extraction studyJ Endod 2006 32:337-40. [Google Scholar]
[3]. Bargholz C, Perforation repair with mineral trioxide aggregate: a modified matrix conceptInt Endod J 2005 38:59-69. [Google Scholar]
[4]. Hashem AAR, Hassanien EE, Pro Root MTA, MTA-Angelus and IRM used to repair large furcation perforations: sealability studyJ Endod 2008 34:59-61. [Google Scholar]
[5]. Ferris DM, Baumgartner JC, Perforation repair comparing two type of mineral trioxide aggregateJ Endod 2004 30:422-24. [Google Scholar]
[6]. Seltzer S, Sinai I, August D, Periodontal effects of root perforations before and during endodontic proceduresJ Dent Res 1970 49:332-39. [Google Scholar]
[7]. Watts JD, Holt DM, Beeson TJ, Kirpatrick TC, Rutledge RE, Effects of pH and mixing agents on the temporal setting of tooth-coloured and gray mineral trioxide aggregateJ Endod 2007 33:970-73. [Google Scholar]
[8]. De-deus G, Petruccelli V, Gurgel-Filho E, Coutinho-Filho T, MTA versus Portland cement as repair material for furcal perforations: a laboratory study using a polymicrobial leakage modelInt. Endo J 2006 39:293-98. [Google Scholar]
[9]. Sarkar NK, Caicedo R, Ritwik P, Moiseyeva RR, Kawashima I, Physicochemical Basis of the biologic properties of mineral trioxide aggregateJ Endod 2005 31:97-100. [Google Scholar]
[10]. Al-Hezaimi K, Naghshbandi J, Oglesby S, Simon JHS, Rotstein I, Comparison of antifungal activity of White coloured and Gray coloured MTA at similar concentrations against Candida albicansJ Endod 2006 32:365-67. [Google Scholar]
[11]. Camilleri J, Ford TRP, Review, Mineral trioxide aggregate: a review of the constituents and biological properties of the materialInt. Endo J 2006 39:747-54. [Google Scholar]
[12]. Torabinejad M, Chivian N, Clinical applications of Mineral Trioxide AggregateJ Endod 1999 25:197-205. [Google Scholar]
[13]. Hardy I, Liewehr FR, Joyce AP, Agee K, Pashley DH, Sealing ability of one-up bond and MTA with and without a secondary seal as furcation perforation repair materialsJ Endod 2004 30:658-61. [Google Scholar]
[14]. Matt GD, Thorpe JR, Strother JM, McClanahan SB, Comparative study of white and gray Mineral trioxide aggregate simulating a One- or Two Step Apical Barrier TechniqueJ Endod 2004 30:876-79. [Google Scholar]
[15]. Nakata TT, Bae KS, Baumgartner JC, Perforation Repair comparing Mineral Trioxide Aggregate and amalgam using an anaerobic bacterial leakage ModelJ Endod 1998 24:184-86. [Google Scholar]
[16]. Fischer EJ, Arens DE, Miller CH, Bacterial Leakage of Mineral Trioxide Aggregate as compared with Zinc-Free Amalgam, Intermediate Restorative Material, and Super –EBA as a Root-end filling materialJ Endod 1998 24:176-79. [Google Scholar]
[17]. Komabayashi T, Spangberg LSW, Comparative analysis of the particle size and shape of commercially available Mineral Trioxide Aggregates and Portland cement: A Study with a flow particle image analyzerJ Endod 2008 34:94-98. [Google Scholar]
[18]. Nekoofar M.H, Adusei G, Sheykhrezae MS, Hayes SJ, Bryant ST, The effect of condensation pressure on selected physical properties of MTAInt. Endo J 2007 40:453-61. [Google Scholar]
[19]. Komabayashi T, Spangberg LSW, Particle size and shape analysis of MTA finer fractions using Portland cementJ Endod 2008 34:709-11. [Google Scholar]
[20]. Al-Hezaimi K, Naghshbandi J, Oglesby S, Simon JHS, Rotstein I, Human saliva penetration of root canals obturated with two types of Mineral trioxide aggregate cementJ Endod 2005 31:453-56. [Google Scholar]