Periodontal disease is one of the most prevalent afflictions affecting the human being [1]. If untreated, tooth may become mobile and finally may get extracted or exfoliated. The ultimate goal of periodontal therapy is creation of an environment that is conducive to maintaining the patient’s dentition in health, comfort and function. Periodontal therapy is directed not only at inflammation control but also at pocket reduction and associated bone defect. The main therapeutic approaches for periodontal diseases include mechanical scaling and root planning, open flap debridement, osseous resection and thus shifting the pathogenic micro-biota to one compatible with periodontal health. Because of the complex anatomy of the roots and the contours of the lesions, the mechanical conventional periodontal treatment alone is not effective in providing optimum periodontal health. The shift in therapeutic concepts from resection to regeneration has significantly impacted the practice of periodontology. Bone replacement grafts have been shown to produce greater clinical bone defect fill from flap debridement alone. In most of the clinical studies, the osseous grafted sites have outperformed the non grafted sites in the regeneration of the lost periodontal structure [2]. The four major types of grafts involved in the treatment of periodontal osseous defects are autogenous, allogenic, xenograft and alloplastic implants. Among the regenerative materials autologous bone seems to be the gold standard but its use is limited by the necessity of an additional surgical procedure, the difficulty of obtaining large amounts of donor tissue and the risk of complications [3]. These advantages have led to the development of myriad grafting materials i.e. Allografts. Allografts, though possesses regenerative properties but due to their side effects like antigenicity and disease transmission, there usage has been limited. These side effects led to the use of xenogratfs as an alternate graft material. The disadvantage of xenograft is that they are bovine in origin and carry the theoretical risk of transmission of bovine spongiform encephalopathy.
The research further led to the development of alloplastic materials which include calcium phosphate, coral & algae derived hydroxyapatite and HTR polymer. The advantages of alloplastic grafts include an absence of antigenicity, no potential for disease transmission and unlimited supply. Alloplastic materials are provided in various particle or pore sizes and can be combined with various carriers to improve handling characteristics.
Alloplastic materials such as hydroxyapatite has shown to have great potential as bone graft material. Tri calcium phosphate (Ca3(PO4)2 which is also known as whitlockite, occurs in alpha and beta phases. Tricalcium phosphate has been shown to have no adverse effect on cell count, viability and morphology and can provide a matrix that favours limited cell proliferation in vitro [4].
Materials and Methods
Ten study subjects, four males and six females, amongst the patients visiting the Department of Periodontology and Oral Implantology in National Dental College and Hospital, Derabassi, Mohali, Punjab were included in this randomized clinical study. The time period of study was six months (June to November 2009) for each patient. An informed consent to participate in this study was obtained from each patient. Also, this study was duly approved by the ethical committee of National Dental College, Derabassi, Mohali, Punjab. The selected subjects were then divided into two groups:-
Group I (PD>7mm).
Group II (PD≤ 7mm).
Inclusion Criteria
Co-operative patients, with one or more vertical defects, between age group of 30-65 yrs of age were recruited for this study. Systemically healthy patients, irrespective of whether they smoked or not, with pocket probing depth of equal to more than 6mm and depth of bone defect equal to or more than 4mm were taken into consideration. The bone defects selected were three walled or two walled and both males and females were included in the study.
Exclusion Criteria
Patients with history of chronic systemic disease, bruxism, on any immunosuppressant drugs, on antibiotic therapy in the past three months prior to the study and who had undergone any periodontal surgical procedure within 6 months prior to this study were excluded from the study. Teeth with three degree mobility, furcation involvement, endodontic lesions, one walled defects and third molars were also ruled out of the study.
| S.no. | Clinical parameters | Method of Assessment |
|---|
| 1 | Pocket depth (PD) | Measured with a William’s graduated Periodontal probe. It was measured as the distance from the free gingival margin to the base of the periodontal pocket. |
| 2 | Clinical attachment level (CAL) | Measured with the help of customized acrylic stent. It is the distance from lower margin of stent to the base of the pocket. Position marked by a groove on prepared acrylic stents, assures reproducible positioning of the probe. |
| 3 | Plaque Index (PI) (Silness and Loe 1964) | Surfaces scored: mesiofacial, midfacial, distofacial and lingual |
| 4 | Gingival index(G.I) (Loe and Silness 1963) | Surfaces scored: mesiofacial, midfacial, distofacial and lingual. |
| 5 | Radiographic bone fill | Radiograph of each experimental site was taken with an intra-oral periapical X- ray, with mesh attached to it, using long cone paralleling technique. It consist (1mm x 1mm) small boxes and bone fill is measured by counting these boxes. |
The above mentioned parameters were recorded at various time intervals and were subjected to statistical analysis [Table/Fig-2,3,4,5,6].
Comparison of percentage (%) change for probing pocket depth between two groups
| N | Group | Mean | Standard Deviation | Mann-Whitney U Test | p-value | Result |
|---|
| %Change baseline to 3months | I | 24.4444 | 1.24226 | 0.000 | 0.016 | S |
| II | 30.1587 | 2.74929 |
| %Change baseline to 6month | I | 39.1667 | 6.31906 | 0.000 | 0.021 | S |
| II | 24.6032 | 6.87322 |
| %Change 3-6months | I | 19.5238 | 7.78830 | 0.000 | 0.021 | S |
| II | -8.3333 | 14.43376 |
S-Significant NS -Non Significant
Comparison of percentage (%) change for clinical attachment level between two groups
| Group | Mean | Standard Deviation | Mann-Whitney U Test | p-value | Result |
|---|
| %Change baseline to 3months | I | 10.0404 | 7.10726 | 3.000 | 0.174 | NS |
| II | 14.5370 | 4.78176 |
| %Change baseline to 6month | I | 17.8586 | 3.85324 | 5.500 | 0.525 | NS |
| II | 21.9444 | 10.55190 |
| %Change 3-6months | I | 8.2222 | 8.44737 | 7.000 | 0.874 | NS |
| II | 8.3333 | 14.43376 |
S-Significant NS -Non Significant
Comparison of percentage(%) change for plaque score between two groups
| Group | Mean | Standard Deviation | Mann-Whitney U Test | p-value | Result |
|---|
| %Change baseline to 3months | I | 35.6863 | 5.26134 | 3.500 | 0.225 | NS |
| II | 23.1373 | 20.72504 |
| %Change baseline to 6month | I | 31.4510 | 14.81761 | 3.500 | 0.219 | NS |
| II | 22.0588 | 9.18382 |
| %Change 3-6months | I | -7.0000 | 22.80351 | 7.000 | 0.879 | NS |
| II | -4.4118 | 18.77521 |
S-Significant NS -Non Significant
Comparison of percentage (%) change for gingival index between two groups
| Group | Mean | Standard Deviation | Mann-Whitney U Test | p-value | Result |
|---|
| %Change baseline to 3months | I | 43.2353 | 17.21419 | 6.500 | 0.763 | NS |
| II | 37.5817 | 25.98193 |
| %Change baseline to 6month | I | 30.6863 | 17.9159 | 4.500 | 0.365 | NS |
| II | 26.4706 | 8.71392 |
| %Change 3-6months | I | -24.5714 | 17.44964 | 6.000 | 0.645 | NS |
| II | -33.3333 | 57.73503 |
S-Significant NS -Non Significant
Comparison of percentage change between two groups
| Group | Mean | Standard Deviation | t | P Value | Result |
|---|
| Percent change baseline (RD1) - radiographic depth at 3 months (RD2) | >7 | 66.0000 | 17.38454 | 1.634 | .153 | NS |
| <=7 | 45.0000 | 18.02776 |
| Percent change baseline (RD1) - radiographic depth at 6 months (RD3) | >7 | 70.0000 | 11.05542 | -.109 | .917 | NS |
| <=7 | 71.1111 | 18.35857 |
| Percent change radiographic depth at 3 months (RD2) - Radiographic depth at 6 months (RD3) | >7 | 4.0000 | 8.94427 | -3.659 | .011 | S |
| <=7 | 26.1111 | 6.73575 |
| Percent change bone fill 3 months to 6 months | >7 | -10.0000 | 22.36068 | 2.925 | .026 | S |
| <=7 | -66.6667 | 33.33333 |
MATERIAL- OSSIFI (β Tricalcium Phosphate plus Hydroxyapatite)
OSSIFI (Regenerative bone matrix) was used in periodontal infrabony defects. Two modes of bone bonding to implanted HA were identified: (1) bone tissue components bonded to hydroxyapatite (HA) via a recrystallization zone similar in structure to the reprecipitation layer in the corrosion assay, and (2) bone tissue components bonded directly to HA crystals with no morphologically discernible signs of dissolution embarrassment. The formation of the bone/HA bond seems to involve dissolution/reprecipitation phenomena (Frank B. Bagambisa 2004). The biologic similarity to natural bone results in optimal biocompatibility and ensures that it is well tolerated by the body [5]. Moreover, it is claimed to be a unique bi- phasic hydroxyapatite and beta tricalcium phosphate having a bioceramic matrix that is inert and highly osteoconductive [6].
Study casts were made for fabrication of acrylic stents, a radiograph of the experimental site was taken with an intra-oral periapical X-ray, with mesh attached to it (Hagen Werker, Germany), using long cone parallel technique. Patients underwent initial therapy consisting of scaling and root planing and oral hygiene instructions. Patients were then sent for routine blood examination.
After seven days of initial therapy, surgical therapy was planned for the patient. Patients were prepared, anaesthetized and draped according to standardized aseptic approach. Under 2% Lignocaine, with adrenaline in Concentration of 1:200000, buccal and lingual full thickness mucoperiosteal flaps were elevated following sulcular incisions, interdental incision and vertical incision, where desired, with help of Bard Parker blade number #12 and number #15. The intraosseous lesions were exposed and the defects were debrided off granulation tissue. Any remaining deposits were carefully removed, and the exposed root surfaces were thoroughly planed with a curette until a smooth hard surface was felt. The bone defects were curetted to remove granulation tissue and care was taken not to injure/remove any part of crest of intrabony defect. The surgical site was then thoroughly irrigated with sterile saline and carefully inspected to ensure complete removal of granulation tissue. The method of inserting the material into the defects was by mixing it to a paste with some of the patient’s own blood, which was withdrawn from the operative site with a cotton pellet. The resultant mixture was taken to the bone defect with the help of plastic instrument. The material OSSIFI (HA+β-TCP) was manipulated according to the manufacturer’s instructions. Graft material was placed, condensed and shaped such that it was lightly packed without overfilling the defect, but restored ideal bone architecture. The flaps were then sutured over the wound with simple interrupted sutures using 3-0 black silk suture. The operated site was then protected with periodontal (Coe-Pak) dressing for a period of one week. At one week postoperative patients returned for re-evaluation dressing changed if necessary.
Results
Pocket depth- The Mean percentage change was calculated [Table/Fig-2,7] and a statistically significant finding in Group II (PD≤7mm) occurred after three months (p=0.016), after six months (p=0.021) compared to baseline and also significant finding was seen when reading after three months was compared with six months (p=0.021).
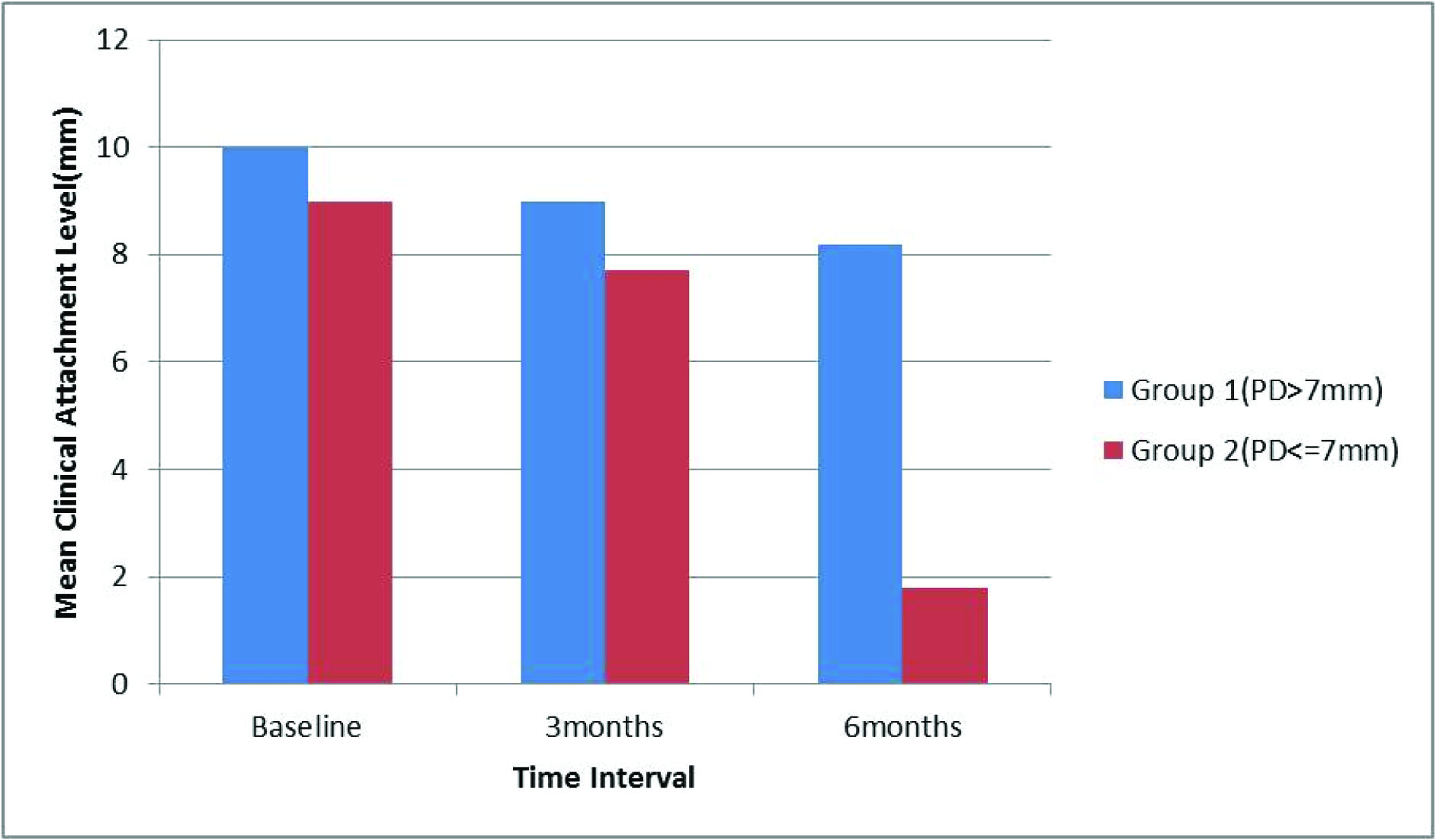
Clinical attachment level- The Mean percentage change was calculated [Table/Fig-3,8] and no statistically significant value, in both the groups, was observed at any visit compared to baseline.
Plaque Index- The Mean percentage change was calculated [Table/Fig-4,9] and no statistically significant value, in both the groups, was observed at any visit compared to baseline.
Gingival Index- The Mean percentage change was calculated [Table/Fig-5,10] and no statistically significant value, in both the groups, was observed at any visit compared to baseline.
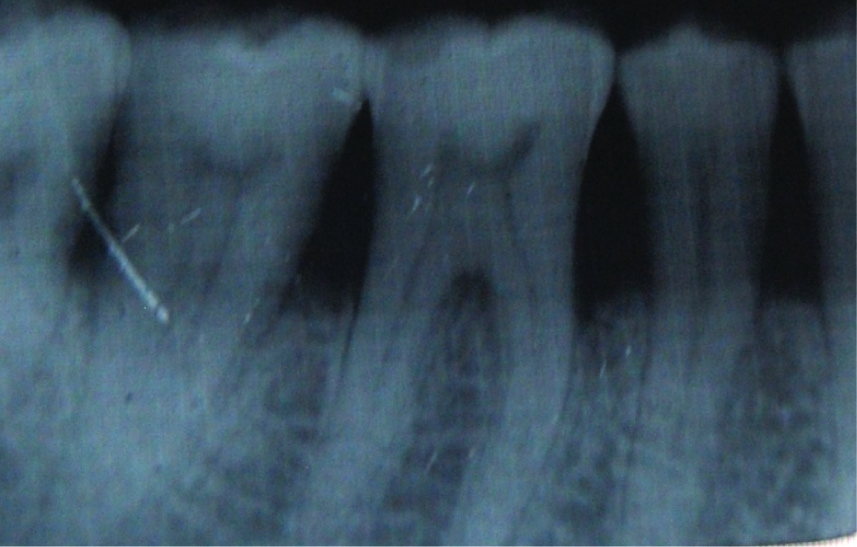
Radiographic Bone Fill - In Group I (PD>7mm), the Mean percentage change was calculated [Table/Fig-6,11] and was significant between three months to six months (p=0.011). In Group II (PD≤7mm), the Mean percentage change was calculated and a statistically significant bone fill occurred between 3-6 mnth reading (p=0.020. The radiographic bone fill is evident in the radiograph as shown in [Table/Fig-12&13].
Mean probing pocket depth at different intervals of time in both groups

Mean clinical attachment level at different intervals of time in both groups
Mean plaque index at different intervals of time in both groups

Mean gingival index at different intervals of time in both groups

Radiographic bone fills in two groups

Baseline radiograph showing intrabony bone defect between first molar and second premolar

Discussion
Today regenerative therapy attempts to focus mainly on two main approaches for periodontal regeneration. First, it’s the introduction of a filler material into the defect in hope of inducing bone regeneration. Secondly, techniques are being developed to guide and instruct the specialized cellular components of the periodontium to participate in the regenerative process.
The change in mean fill from baseline to three months & baseline to six months was statistically significant (p=0.002, p=0.001). Between three months and six months, mean bone fill for Group I was observed to be 0.20±0.44 and for Group II it was observed to be 0.13±0.57. Radiographically bone fill was seen but no statistically significant results were observed. The osseointegrative effect of the graft is attributed to its chemical structure and was suggested by Shetty S et al.,[7] that hydroxyapatite acts as an amphometric ion exchanger. Selective accumulation of calcium and phosphate ions occurs as a consequence of the negative charges on the hydroxyapatite surface. This leads to the formation of more apatite and stimulates the formation of new bone. Min-Jae Lee et al., [8] found similar results when they compared the clinical outcome of open flap debridement (OFD) with a biphasic calcium phosphate (BCP) graft to that of OFD without BCP graft for the treatment of intrabony periodontal defects (IBDs). Attila Horvath et al., [9] clinically and histologically evaluate the healing of human intrabony defects treated with open flap surgery (OFD) and application of a new, resorbable, fully synthetic, unsintered, nanocrystalline, phase-pure hydroxyapatite.
In the present study the mean value of clinical attachment level at six months interval reduced to 8.20±0.44mm and 7±1.00mm respectively postsurgically. Between three months to six months mean clinical attachment level observed for Group I(PD>7mm) was 1.63±0.44 and for Group II(PD≤7mm) to be 1.00. These results are in accordance with the study performed by Adrian Kasaj et al., [10], who reported that hydroxyapatite paste when used in treating periodontal defects resulted in change in mean clinical attachment level from 8.1mm at baseline to 6.4mm at six months, post surgically. Similar results were seen by Saini S et al., [11] in which all three groups showed statistically significant PPD reduction, CAL gain, DF, and %DF when they determined Efficacy of combination techniques using TCP+CA; TCP+CA+ORC in treatment of periodontal infrabony defects.
In the present study, mean probing pocket depth reduction of 5.00±0.70mm was seen for Group I compared to 5.00±0.00 for Group II after six months. Between three months to six months mean probing pocket depth observed for Group I (PD>7mm) was 2.12 and for Group II (PD≤7mm) to be 1.00. This mean reduction has been attributed to the osteogenic potential of the bone graft that has been used for this study. These results are in accordance with the results of Kasaj A et al., [10], who reported substantially improved pocket depth with the use of hydroxyapatite paste in the treatment of human periodontal bone defects. They showed a reduction in mean pocket depth from 7.4mm at baseline to 3.4mm at 6months following surgery. Sculean et al., [12] clinically and hisologically evaluated the combined use of enamel matrix derivative and biphasic calcium phosphate in the treatment of human intrabony defects and demonstrated a mean probing depth reduction from 8.6mm at baseline to 5.3mm, post surgically after nine months. Thus these results correlated with results obtained in this study.
In the present study, reduction in mean plaque score was observed to be 1.62±0.10 at baseline to 1.12±0.29 after 6months, for Group I and from 1.8 ±0.17at baseline to 1.4±0.17 after six months for Group II. The reduction in plaque score was attributed to the continuous reinforcement of oral hygiene instructions from time to time follow up protocol. Srikanth et al., [13] evaluated the efficacy of hydroxyapatite granules in the treatment of human periodontal defects and demonstrated the change in mean plaque index from 0.13 at baseline to 0.70 after six months with no statistically significant results.
In the present study mean gingival index for Group I and group II reduced to 1.02±0.20 and 1.46±0.25 respectively at six months. Statistically significant gingival index was observed only in Group I with p=0.039 for first three months and was non-significant for later duration. No significant results were obtained for Group II patients [Table/Fig-12]. These low scores were due to the proper compliance of the patient to oral hygiene instructions that were reinforced at the recall visits. Dori et al., [14] reported similar results in their study evaluating the enamel matrix with natural bone mineral or β CP. Gingival index improved clinically compared to baseline but no statistically significant results were obtained. Rupprecht et al., [15] evaluated hydroxyapatite in the treatment of class III furcation defects and demonstrated reduction in gingival index from 1.3±0.5 at baseline to1.0±0.0 at re-entry.
Conclusion
The decision to treat a defect with a regenerative technique must be based on consideration depth and width of defect and attachment level prior to treatment, thickness of the gingival flap, plaque accumulation, quality of the recall maintenance programme, periodontal history of the affected tooth, healing response of the subject and clinicians surgical skill which in turn will determine the predictability of a successful result. However, findings for some of the parameters are highly significant but these findings cannot be taken as affirmative in study. To reach at a reasonable level of confirmation about the efficacy of material, long term studies and an increased sample size is indicated.