Periodontal diseases are chronic inflammatory disorders comprising of many diseases out of which gingivitis and periodontitis are the most common. These occur as a result of the interaction between a pathogenic bacterial biofilm and host-derived inflammatory cells and molecules [1,2]. Gingivitis is a reversible inflammatory condition while periodontitis is a chronic irreversible inflammatory state of the supporting structures leading to destruction of connective tissue and alveolar bone [3,4]. The traditional methods for diagnosis of these diseases involve clinical measurements and radiographic assessments which are often poorly tolerated by the patients and are also subjected to measurement errors [2]. Hence, today various researches are being conducted to evaluate possible compounds in the oral fluids through which it may be possible to assess the presence and severity of these diseases as well as to identify the patients at risk for these diseases [2].
Saliva is an oral fluid which can be easily and rapidly collected and does not require any specialized equipment or techniques [4]. One of the components of saliva are the salivary mucins which play a major role in modulation of oral flora, lubrication and tissue coating of oral hard and soft tissues [5]. Recent studies indicate that salivary mucin exhibits bactericidal activity against Aggregatibacter actinomycetemcomitans which is among the major pathogens responsible for periodontitis [6]. Salivary amylase also exhibits inhibitory activity against various microorganisms [5]. Also, patients with periodontal disease have differences in the protein composition of whole saliva [2].
So far there is sparse literature regarding the relationship between mucin, amylase, total protein and gingivitis and periodontitis. Hence, this case-control study was designed to assess and compare the salivary levels of mucin, amylase and total protein in healthy, gingivitis and chronic periodontitis subjects.
Materials and Methods
The present study was conducted on 90 individuals of age between 25-60 years in the Department of Periodontics, A.B. Shetty Memorial Institute of Dental Sciences, Mangalore, India. The study group consisted of 30 individuals with gingivitis, 30 individuals with chronic periodontitis (CP) and 30 age and gender matched healthy individuals (controls). The groups were divided based on the condition of the periodontal tissues which was evaluated both by clinical as well as by radiographic examination. A complete periodontal examination included assessment of probing pocket depth (PPD), clinical attachment loss (CAL), gingival index (GI) (Loe and Silness) [7] and panoramic radiographs. PPD and CAL were measured at six sites per tooth (mesio-, mid-, disto-buccal/palatal or lingual).
Inclusion criteria for the study included: subjects with minimum complement of 20 natural teeth, Controls with a GI score ≤ 1 and absence of loss of attachment. In the gingivitis group, subjects with bleeding on probing and GI score ≥ 1 in more than 30% of sites were included. Subjects having clinical attachment loss of ≥ 4mm with radiographic evidence of bone loss in more than 30% of the sites were included in the CP group.
Exclusion criteria included subjects with history of any systemic diseases or conditions, subjects with history of any salivary gland diseases, subjects with any apparent oral infections (i.e. herpes or candida) or injuries or bleeding in the oral cavity unrelated to gingivitis or periodontitis, intake of antibiotics or anti- inflammatory drugs within 6 months prior to the study, pregnant or lactating women, subjects with history of smoking or any form of tobacco and alcohol consumption. Individuals who had received any periodontal treatment in the past six months prior to the study were also excluded from the study. The study was conducted according to the provisions of Helsinki Declaration of 1975 revised in 2000 and was approved by Institutional ethics committee. Written informed consents were obtained from the subjects.
Collection of Saliva
Subjects were instructed not to brush their teeth, or eat or drink one hour before the time of saliva collection. Unstimulated whole saliva was collected between 11am and 12 Noon to avoid diurnal variation with patients seated with instructions to allow saliva to accumulate in the floor of the mouth and to spit without stimulation into the sterile container. The collected samples were immediately taken for biochemical analysis.
Amylase estimation was done by Kinetic method. 25μl of saliva sample and 1000μl of the reagent (ready to use liquid kit) was mixed and incubated at 37oC. After 15sec, it was measured and absorbance was taken at 405nm. Two additional absorbance was taken at 30sec interval. The mean absorbance change per minute was calculated (ΔA/min) and multiplied by factor 4824.
Mucin estimation was performed using Alcian Blue method [5,8,9]. Briefly, saliva samples were taken in test tubes and diluted with distilled water. Then 1% solution of Alcian blue in 50mM sodium acetate buffer was added and was incubated at room temperature under continuous agitation for 30min. After incubation, samples were centrifuged for 20min at 3000 rpm. Then one ml of 95% ethanol was added and vortexed for 10sec. After 5min, the samples were centrifuged for 20min at 3000 rpm. Then Aerosol OT (1:2 dilutions with distilled water) was added followed by addition of equal quantity of ethyl ether under vigorous shaking. The samples were centrifuged for 15min at 3000 rpm and then the optical density was measured at 605nm. Concentration of mucin was then calculated from the standard graph.
Total protein was evaluated by Lowry method [10]. Briefly, 0.2ml of Bovine serum albumin (BSA) working standard was taken in five test tubes and made up to 1ml using distilled water. The test tube with 1ml distilled water served as blank. 4.5ml of Reagent I (48 ml of 2% Na2CO3 in 0.1 N NaOH, 1ml of 1% NaK Tartarate in water, 1ml of 0.5% CuSO4.5 H2O in water) was added and incubated for 10min. After incubation 0.5ml of Reagent II (1 part Folin Ciocalteu’s phenol reagent (2 N): 1 part water) was added and incubated for 30 minutes. The absorbance was measured at 660 nm and the standard graph was plotted. The amount of protein present in the given sample was estimated from the standard graph.
Statistical Analysis
The data obtained were subjected to statistical analysis using SPSS software. The values obtained were tabulated and mean and standard deviation of each measured parameter was calculated. The test of significance applied was ANOVA (Analysis of Variance) test to compare the levels of mucin, amylase and total protein in saliva of subjects with healthy periodontium, gingivitis and chronic periodontitis. Correlation between mucin, amylase and total protein levels in saliva was carried out using Karl pearson’s correlation test. p-value ≤ 0.05 was considered as statistically significant.
Resutls
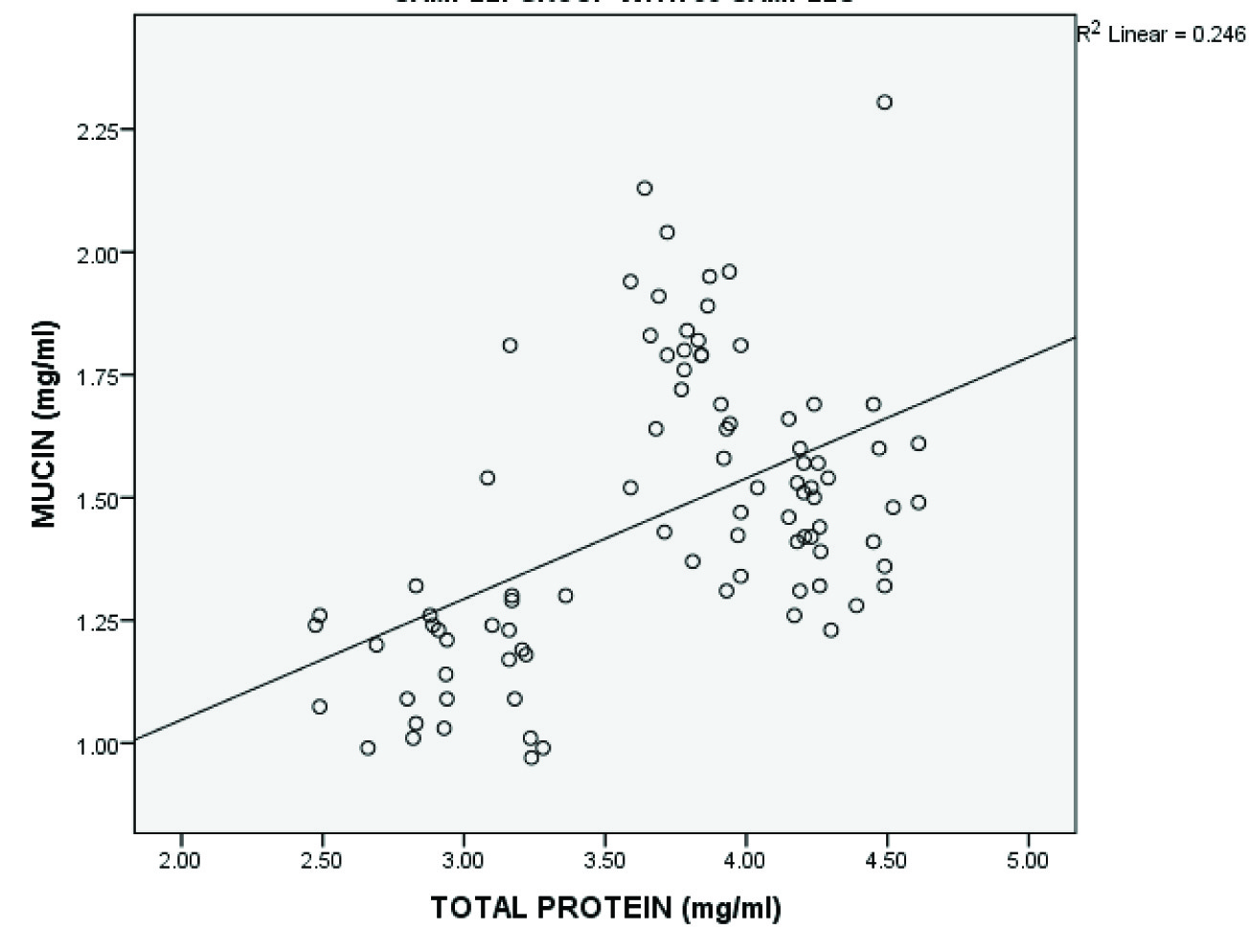
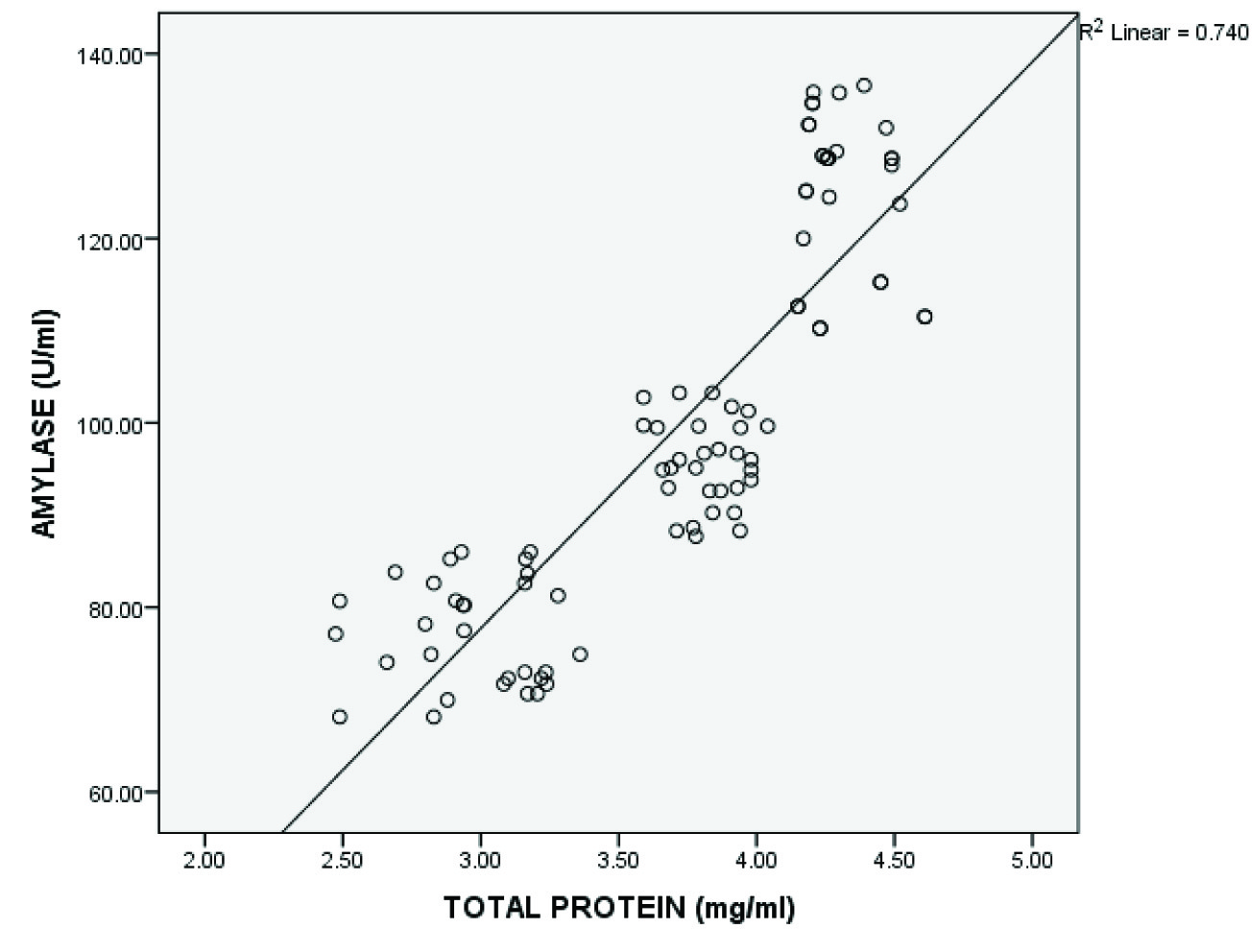
The study showed an increased concentration of salivary mucin, amylase and total protein in gingivitis patients and increased levels of amylase and total protein in saliva of chronic periodontitis patients compared to healthy individuals which were statistically significant. A decrease in mucin concentration was observed in the periodontitis group compared to gingivitis group [Table/Fig-1,2,3,4]. A statistically significant positive correlation was present between salivary mucin, amylase and total protein levels in the three groups [Table/Fig-5,6,7,8].
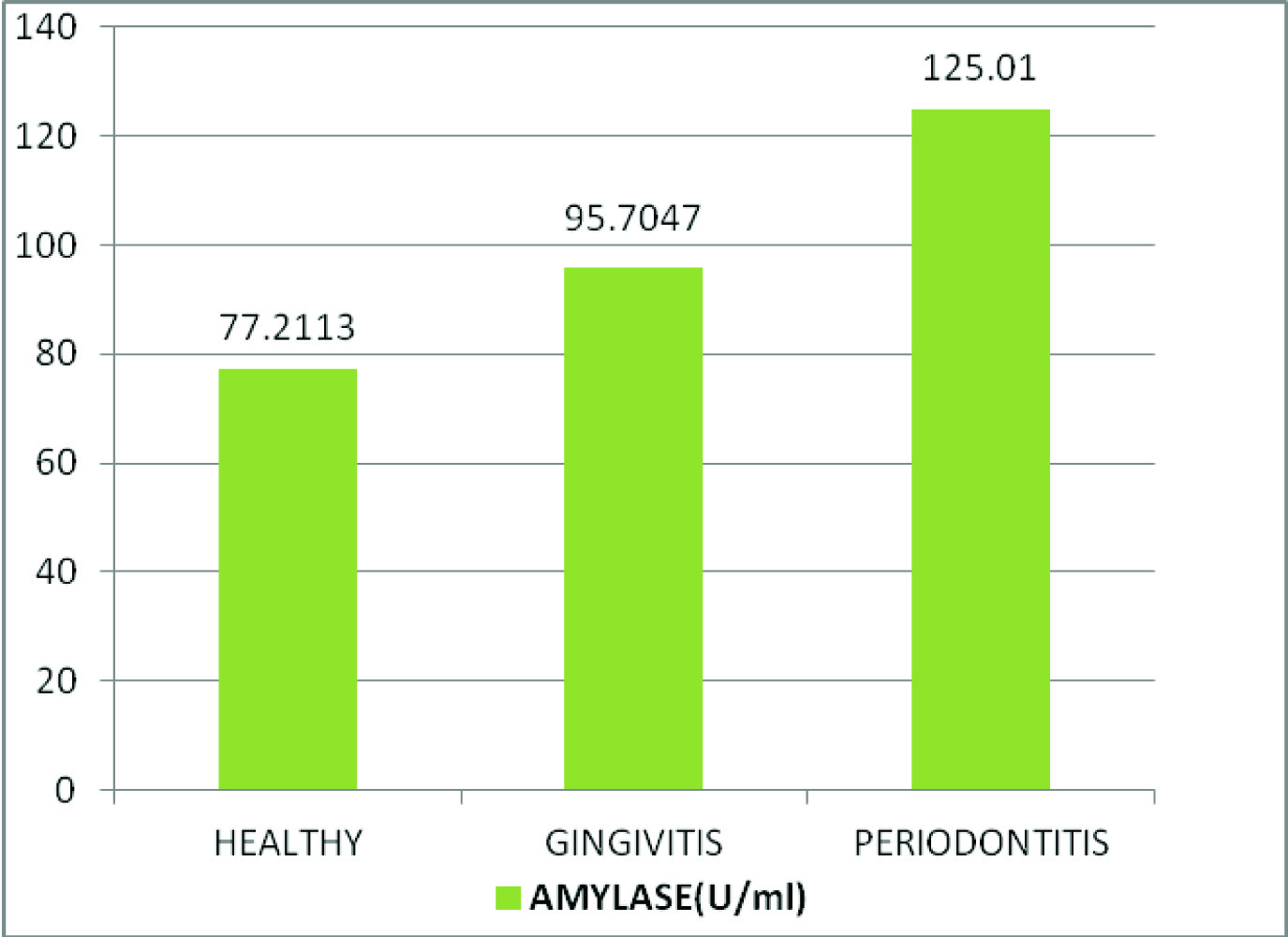
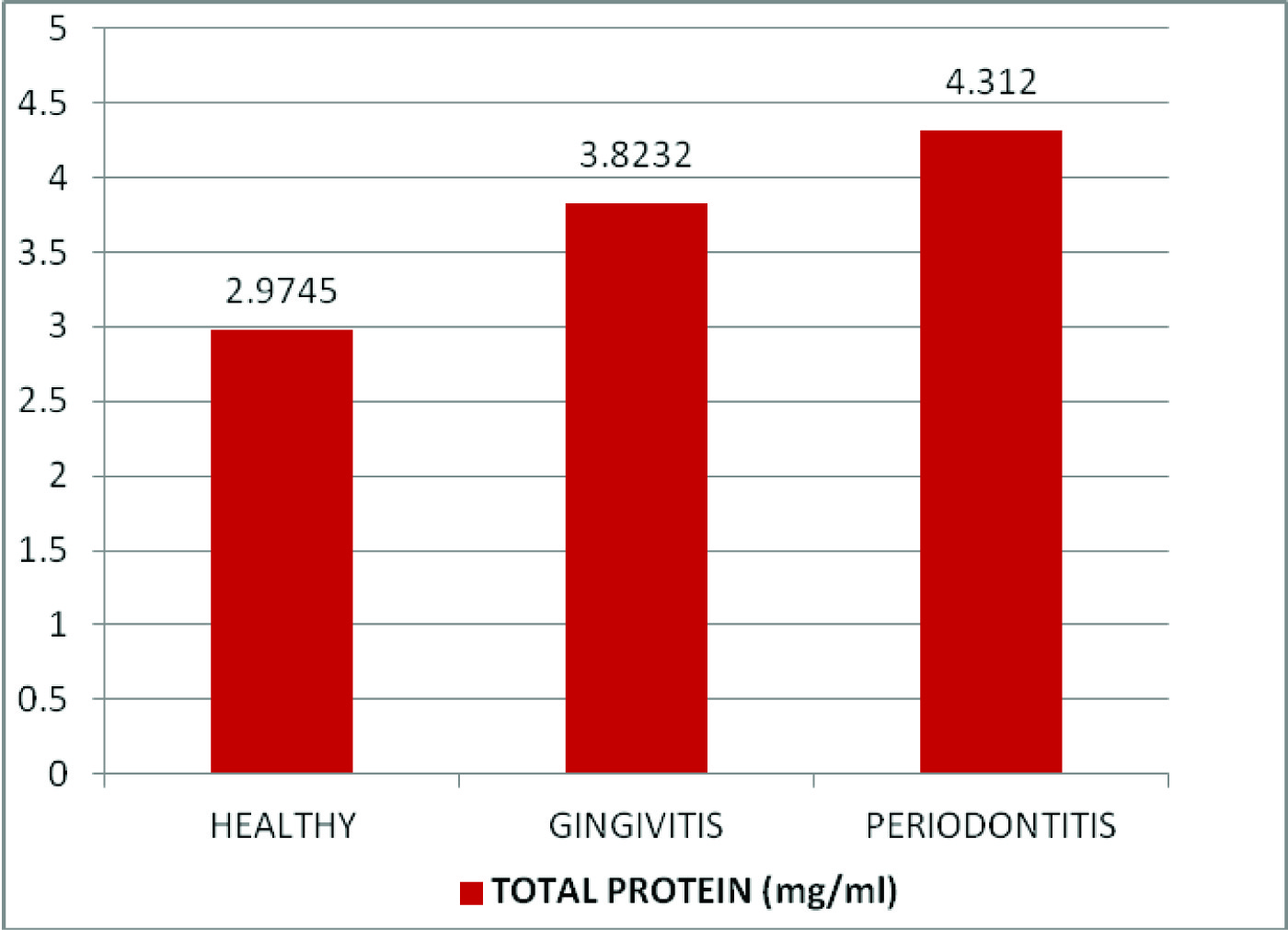
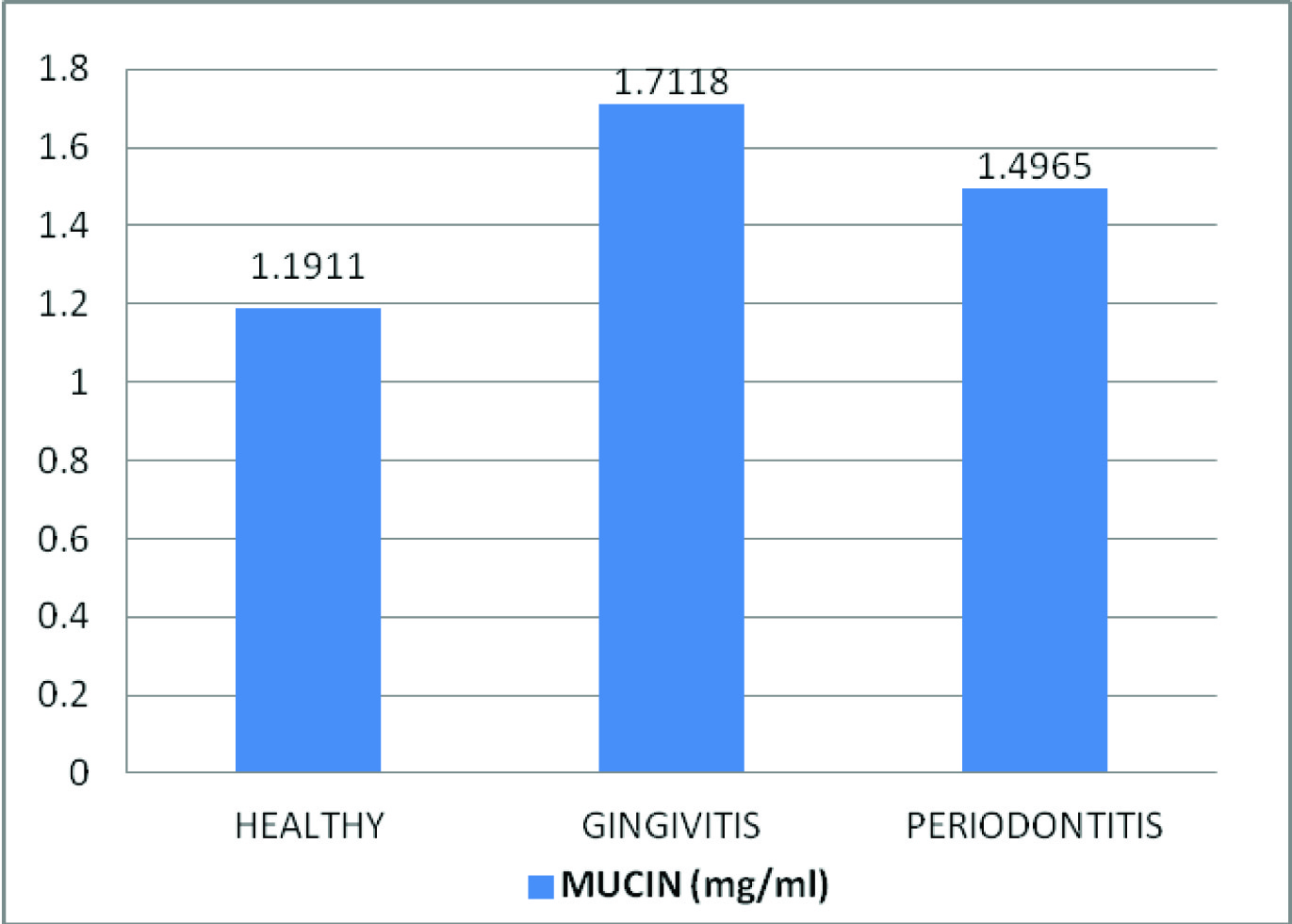
Comparison of mean salivary amylase, total protein and mucin levels between the control and experimental groups
| Group | n | Mean | SD | ANOVA test result -f value | p value |
|---|
| Amylase (U/ml) | Healthy | 30 | 77.2113 | 5.74934 | 55.285 | <0.001* |
| Gingivitis | 30 | 95.7047 | 4.67199 |
| Chronic Periodontitis | 30 | 125.01 | 8.62857 |
| Total protein (mg/ml) | Healthy | 30 | 2.9745 | 0.24699 | 55.152 | <0.001* |
| Gingivitis | 30 | 3.8232 | 0.1264 |
| Chronic Periodontitis | 30 | 4.312 | 0.14299 |
| Mucin (mg/ml) | Healthy | 30 | 1.1911 | 0.17288 | 53.308 | <0.001* |
| Gingivitis | 30 | 1.7118 | 0.21557 |
| Chronic Periodontitis | 30 | 1.4965 | 0.19797 |
* Very highly significant (Level of significance p-value ≤ 0.05)
Comparison of mean salivary amylase levels (U/ml) between healthy, gingivitis and chronic periodontitis patients
Comparison of mean salivary total protein levels (mg/ml) between healthy, gingivitis and chronic periodontitis patients
Comparison of mean salivary mucin levels (mg/ml) between healthy, gingivitis and chronic periodontitis patients.
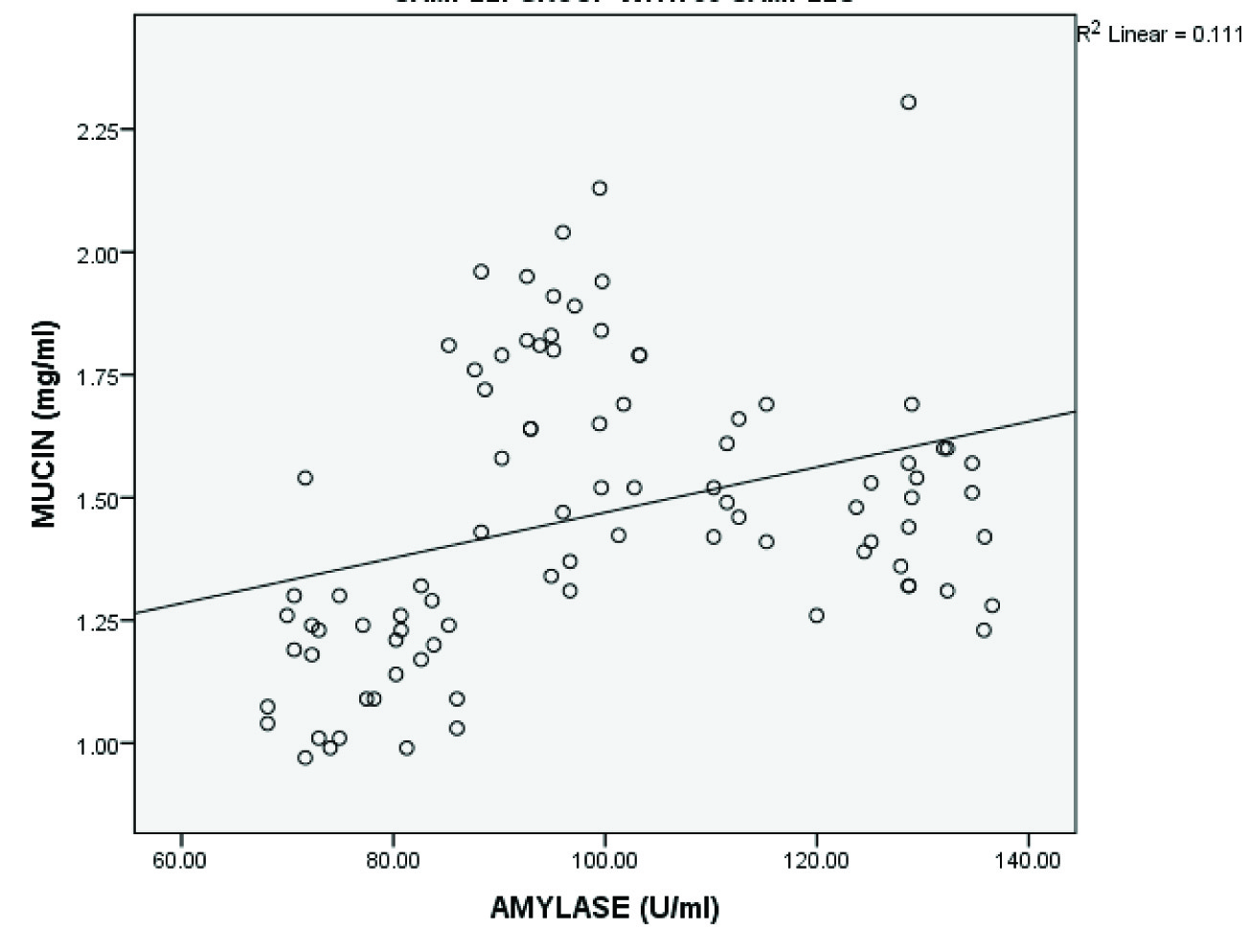
Correlation between mean salivary mucin, amylase and total protein levels in the three groups
| Parameters correlated | N | Pearson correlation (R value) | p value |
|---|
| Salivary mucin and amylase | 90 | 0.333 | 0.001† |
| Salivary mucin and total protein | 90 | 0.496 | <0.001* |
| Salivary amylase and total protein | 90 | 0.860 | <0.001* |
* Very highly significant † Highly significant (Level of significance p value ≤ 0.05)
Shows significant positive correlation between salivary mucin and amylase levels
Shows significant positive correlation between salivary mucin and total protein levels
Shows significant positive correlation between salivary amylase and total protein levels
Discussion
Salivary amylase is a calcium containing metalloenzyme that catalyses the hydrolysis of α[1,4] glycosidic bonding between glucose residues of polysaccharides such as starch, glycogen and dextrins [5,11]. In addition to its digestive action, α-amylase is also said to possess anti-microbial properties and thus may participate in the oral defense mechanism [12].
The results of our study showed a significant increase in the levels of salivary amylase in gingivitis and chronic periodontitis patients as compared to healthy individuals.
The increased levels may be due to the response of salivary glands to inflammatory diseases like gingivitis and periodontitis resulting in increased synthesis and secretion of certain acinar proteins like α-amylase so as to enhance the oral defense mechanism [12,13].
Studies show that α-amylase is a major lipopolysaccharide binding protein of Aggregatibacter actinomycetemcomitans and Porphyromonas gingivalis and interferes with bacterial adherence and biofilm formation [14,15]. Thus, the high concentration of salivary α-amylase seen in our study suggests it to be an important defense molecule essential for the innate immunity in the oral cavity.
Our study is in accordance with other studies which showed an increase in amylase levels in patients with gingivitis and periodontitis [5,13,16,17].
Total protein is a vital component of saliva and is responsible for most of its functions like lubrication, physical protection, cleansing, buffering, maintenance of tooth integrity, taste and digestion and antibacterial activity [2,18].
The results of our study showed higher levels of total protein in gingivitis and chronic periodontitis patients as compared to healthy individuals which was statistically significant.
The increased protein levels in our study in the test groups could be due to the inflammatory process that activates the sympathetic system to enhance the synthesis and secretion of some proteins (as evidenced by increased amylase levels) thereby increasing the protective potential of saliva against the diseases [12,13].
The increased levels could partly be also due to an increased leakage of plasma proteins into saliva due to inflammation as suggested in a study done by Henskens et al., [19].
Our results are in agreement with other studies which showed similar results [12,20].
Mucins contained in human saliva represent a heterogeneous group of glycoproteins synthesized and released by the submandibular and sublingual glands, and small glands of the oral mucosa [21]. Currently, two main groups of salivary mucins are identified: high-molecular weight MG1 (mucin glycoprotein-1) mucins and low-molecular weight MG2 (mucin glycoprotein-2) mucins [22]. These mucins are important both for maintaining the viscoelastic and rheological properties of saliva as well as for binding and facilitating clearance of variety of oral microorganisms [23].
In the present study, an evaluation of mucins in unstimulated saliva was performed in healthy, gingivitis and chronic periodontitis subjects. To our knowledge, this is the first study evaluating mucin levels in saliva of gingivitis patients. The results showed an increase in the levels in gingivitis patients. Similar to total proteins and alpha amylase, the increase in mucin concentration may be considered as an effort of salivary glands to enhance the protective potential of saliva, since mucins has been known as one of the proteins that protects the oral cavity against microbial infections [24].
A decrease in mucin concentration was observed in periodontitis group compared to the gingivitis patients. This can be interpreted in two ways:
Firstly, there may be decreased synthesis or secretion of mucins by the salivary glands. Studies show that in periodontitis, in response to various pro-inflammatory stimuli like cytokines, endotoxins like lipopolysaccharide (LPS), there is increased expression of inducible nitric oxide synthase (iNOS) which leads to production of large quantities of nitric oxide (NO) [25,26]. Exposure of acinar cells of salivary glands to NO causes a dose dependent decrease in synthesis of mucin [27]. LPS of certain bacteria like Porphyromonas gingivalis (P.gingivalis) can also directly act on acinar cells to decrease mucin synthesis [28]. Thus, the use of nitric oxide synthase inhibitors as a part of host modulation therapy may be of great value in the treatment of periodontitis.
Secondly, there may be increased degradation of mucins by the oral microorganisms. During plaque maturation, plaque contains both gram positive and gram negative bacteria, the growth of which is primarily dependent upon their ability to utilize endogenous nutrients, especially glycoproteins, including salivary mucins [29]. Mucin degradation is completely achieved by a combination of saccharolytic and proteolytic enzymes including proteases, glycosidases, sialidases and sulfatases, together designated as “mucinases”[30]. Degradation of mucin in vivo starts with cleavage of non-glycosylated regions of polypeptide backbone by proteolytic enzymes. Subsequently, the oligosaccharide chains are degraded by a variety of glycosidases, sialidases, sulfatases and α-fucosidases released by various microorganisms leading to exposure of the protein core [30]. The exposed protein core of the mucins is further degraded by proteases released by various gram-negative micro-organisms like P.gingivalis [31].
Studies show that sialoglycoproteins (e.g. sialomucins) acts as key in vivo nutrient source for tannerella forsythia (due to its sialidase activity), a key pathogen in chronic periodontitis, when growing in a biofilm [32]. This suggests that various mucinase inhibitors might also be useful as a part of host modulation therapy in the treatment of periodontitis.
Our study is in accordance with the study done by Groenink et al., [33] who showed that MG2 output was decreased in individuals with aggressive periodontitis in comparison with periodontal healthy subjects.
A study done by Rocha et al., [1] also showed reduced expression of MG2 in chronic and aggressive periodontitis patients compared to healthy individuals. Contrary to our findings, some studies showed increased levels of salivary mucin in periodontitis subjects [5,12].
In our study, a significant positive correlation was also observed among proteins, amylase and mucin indicating that they are implicated in host response. This is in accordance with other studies which also showed a positive correlation among proteins, mucin and amylase [5,12].
Conclusion
The study showed increased levels of salivary mucin, amylase and total protein in gingivitis patients and increased levels of amylase and total protein in chronic periodontitis patients while salivary levels of mucin were decreased in periodontitis patients. The results provide evidence that salivary glands may respond to periodontal diseases by enhanced synthesis of some acinar proteins, thereby increasing the protective potential of saliva.
Thus, salivary mucin, amylase and total protein may serve as an important biochemical parameter of inflammation of the periodontium. Also, certain microorganisms may evade the host defense mechanism by causing decreased synthesis of certain proteins or increased degradation of the protein by release of various enzymes. Thus, it can be hypothesized that various enzyme inhibitors might be therefore useful as a part of host modulation therapy in the treatment of periodontal diseases.
* Very highly significant (Level of significance p-value ≤ 0.05)
* Very highly significant † Highly significant (Level of significance p value ≤ 0.05)