Introduction
Oral squamous cell carcinoma (OSCC) is the most common cancer of the oral cavity, accounting for more than 90% of all the oral cancers. Each year, globally, there are 222,000 new cases of oral cancer diagnosed in men (5% of all cancers) and 90,000 new cases diagnosed in women (2% of all cancers) [1]. More than 90% of oral and pharyngeal cancers are squamous cell carcinomas. The other 10% compromise salivary gland tumours, lymphoma, sarcoma and others. The five year survival rate of these cancers has improved only modestly in the past 30 years and remains at approximately 55-60% [2]. Oral cancer is usually first diagnosed when symptoms start to appear. Thus 2/3rds of patients already have advanced disease leading to poor prognosis. For this reason, diagnostic aids for oral cancer have been developed.
The early detection of premalignant lesions of the oral cavity allows for treatment that may be sufficiently early to prevent their progression to an invasive carcinoma thus improving both the survival rate.
The diagnosis of dysplastic pre-malignant lesions of oral mucosa cannot be based solely on clinical findings, and supplementary biopsy with histopathologic examination is necessary. Therefore the diagnosis is based on clinic-pathologic correlation. Hence, a need for non-invasive technique for early detection of cancers is long been recognized [3].
There are numerous techniques developed to facilitate identification of initial carcinomas. Toluidine blue vital staining has been advocated as a simple, inexpensive and sensitive chairside test and is being used most commonly. But in contrast, studies have shown the risk of affinity of the stain with DNA and as high as 30% risk of false-positive staining attributed to enhance the staining of ulcerations and filiform papillae of the tongue [4]. Exfoliative cytology has a false-negative rate of approximately 30% [5]. Other techniques such as autofluorescence, oral brush biopsy, acetic acid staining [4], chemiluminescence [6] etc are however not accurate to establish a final diagnosis.
Dysplasia and carcinoma in situ herald invasive oral cancer, but carcinomas also occur in areas with no previous signs of dysplasia [7]. This may be caused by the rapid emergence of invasive cancer, but it is also possible that earlier biopsy specimens were taken from unrepresentative sites of the lesion or before morphologic changes could be detected [8]. Consequently, the specimen must be taken from the most representative area. Hence, there is a pressing need for a non-invasive, more accurate diagnostic tool. Colposcopy serves this purpose. Colposcopy is a diagnostic procedure to examine an illuminated magnified view of vagina, cervix or oral mucosa with good resolution. Many premalignant and malignant lesions have discernible characteristics which can be detected through this examination, usually done by an instrument known as “Colposcope”. The main goal of colposcopy is to aid in the prevention of the premalignant lesions by early detection and treatment.
Here, we present a review on colposcope and its oral application in early diagnosis of premalignant and malignant lesions of oral mucosa.
Colposcope
Since ages Colposcope has been routinely practiced in Gynecology. It was invented by Prof. Hans Hinselmann [9]. It was only in the year 2000, Goran Gynther used colposcope for oral lesions [10].
A colposcope is typically defined as a stereoscopic binocular field microscope with a long focal length and powerful light source [9]. The parts of a colposcope include a colposcope head, a height adjustment knob to adjust the height of the instrument, head inclination knob to adjust the angulation. Illumination is provided by a halogen lamp via a fibreoptic cable connected to a system of lenses. It can magnify the tissue from 4 to 40 folds. The colposcope head consists of a pair of diapter knobs, 45o angled lens, objective lens, three step magnification knob for low, medium and high power [Table/Fig-1].
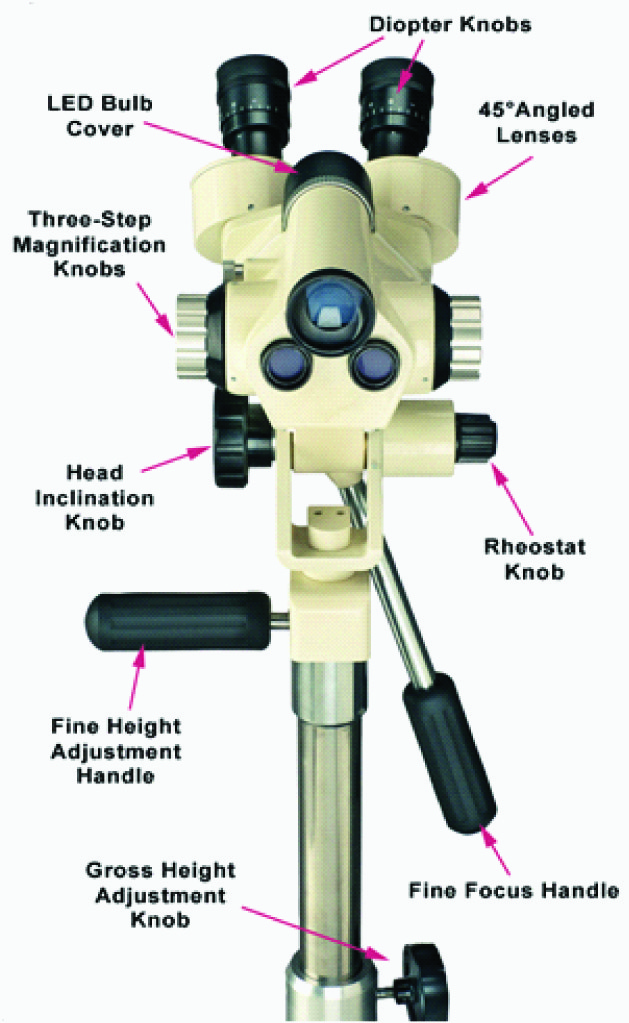
Various light filters are available to highlight different aspects of the surface of the tissue. Colposcope uses a green or blue filter to facilitate the examination of vascular changes and colour tone because the unfiltered white or yellow light reduces the contrast between the terminal vessels and the surrounding tissue. The green filter removes red light thereby enhancing the vascular details by making blood vessels appear dark. The focal length of the microscope is 200 mm, providing an optimal working distance. It provides 3-dimensional image of tissue surfaces examined [9].
Staining Procedure
Acetic acid wash
The sensitivity and specificity of 4% acetic acid in detection of cervical cancer in cervical cancer in India was proved to be 88% and 78% respectively as reported by Sankaranarayanan and colleagues [11]. Since the anatomy of and the types of cancer found in the oral cavity and cervix are comparable, acetic acid seems to be an appropriate clinical marker for the detection of oral cancer as well. 4% acetic acid is applied to the mucosa using cotton swabs for about 30 s. The acetic acid coagulates mucus, which is then easily removed. It produces swelling of both squamous and columnar epithelium and reduces its transparency by producing a transient coagulation of nuclear proteins and perhaps by other mechanisms which are as yet unclear.
The acetic acid does not affect the mature, glycogen-producing epithelium because the acid does not penetrate below the outer one-third of the epithelium [12]. The cells in this region have very small nuclei and a very large amount of glycogen [12]. Dysplastic cells are the ones which are most affected. They contain large nuclei with abnormally large amounts of chromatin. The areas that stain white after the acetic acid wash are called acetowhite lesions. The vascular patterns are clearly seen just as the acetowhitening begins to fade. Areas of tissue which turn white after the application of acetic acid or have an abnormal vascular pattern are often considered for biopsy.
Lugol solution
If after acetic acid application no lesions are visible, Lugol’s solution or Schiller’s solution is used for further examination of abnormalities. Staining of the Lugol’s iodine depends on the glycogen content present in the normal epithelium and this selective character of staining helps in delineating the inflammatory or carcinomatous epithelium from the normal epithelium where the glycogen content is low. Brown stain was considered as positive for lesions while lesions without any retention of stain were considered as negative [12].
The acetic acid solution and iodine solution (Lugol’s or Schiller’s) are applied to the surface to improve the visualization of abnormal areas.
Tumour Progression and Vascular Changes
In a few studies conducted by Dhakal et al.,[13], Pazouki et al.,[14], it was concluded that there was a close relationship between vascularity and tumour progression in oral mucosa. The vascularity changes described in colposcopy can be used for selecting biopsy sites in oral cavity. One of the most frequently used index is the Reids index [Table/Fig-2] [15].
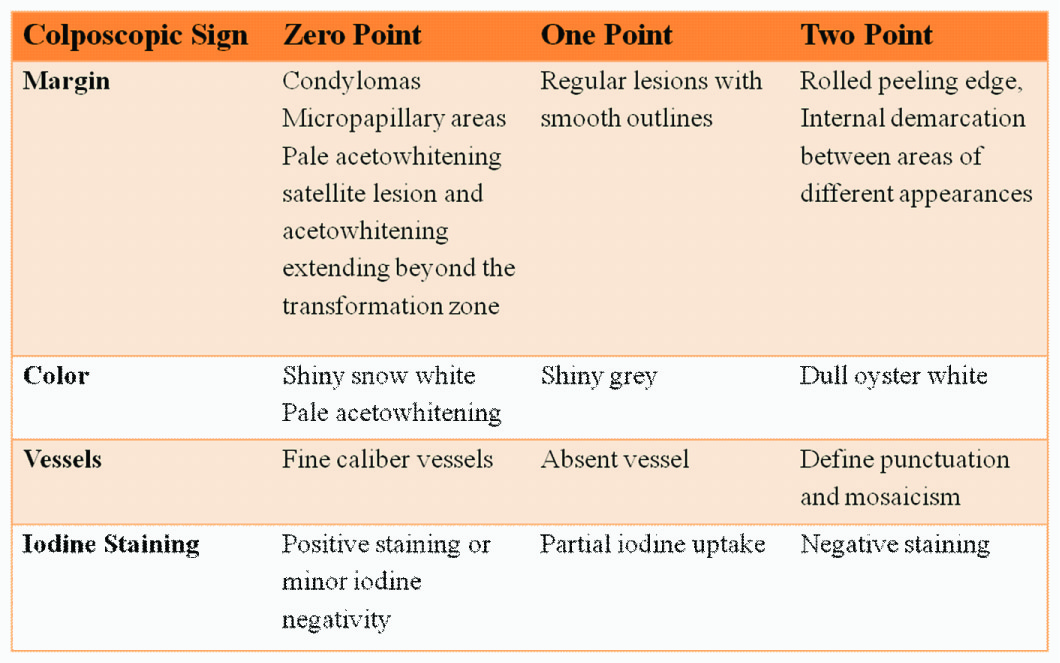
The normal squamous epithelium of the oral mucosa is pink and smooth and it demonstrates as fine, regular vessels. This normal vascularity can be altered in various inflammatory, benign, and malignant lesions and conditions [16]. Colposcopic findings suggesting invasion are: vascular pattern, inter-capillary distance, surface pattern, colour tone and opacity as well as the clarity of demarcation of the mucosal lesions [1]. Because of the increase in vascularity, necrosis of the surface epithelium occurs and in some cases production of keratin leading to colour change. With the application of 3-5% acetic acid, the high grade lesions demonstrate a more persistent duller shade of white, whereas low-grade lesions are translucent or bright white and fade quickly. Low-grade lesions have feathery margins and irregular borders whereas high-grade lesions have straighter, sharper outlines and well-defined borders. A lesion with an internal border that is a lesion within a lesion is typically high grade [17]. These results are based on the vascular and tissue changes. The capillary changes preceding tumour growth with the pattern of tumour angiogenesis are different from the usual neo-vascularization taking place during repair and regeneration processes. At a cellular level, various molecules such as vascular endothelial growth factor, basic fibroblast growth factor, and transforming growth factor alpha are implicated. Direct optical visualization of these patterns would be helpful in the early determination of the underlying pathology and also aid in marking out the site of biopsy [1].
Blood Vascular Pattern: The criteria for vascular changes described in colposcopic literature for the selection of biopsy site has been described as follows [18–21].
Normal: In the normal mucosa, two basic types of capillary networks can be seen with the colposcopy procedure - Network capillaries and Hairpin capillaries [Table/Fig-3].
(A)Illustration of Network capillaries in colposcopic view. (B) Network capillaries in normal buccal mucosa (C) Illustration of hairpin capillaries in colposcopic view. (D) Hair pin pattern in normal buccal mucosa

Abnormal: In the abnormal epithelium, three different types of capillary networks are included.
In areas of dysplasia and carcinoma in situ of the uterine cervix or oral mucosa, a specific vascular pattern, punctuation, is common, characterized by dilated, often twisted, irregular, hairpin-type vessels. These dilated capillaries terminating on the surface appear from the ends as a collection of dots and are thus referred to as punctuation [Table/Fig-4a,b]. Another pattern of the vessels in dysplasia is called mosaic. Terminal capillaries surrounding roughly circular or polygonal blocks of acetowhite epithelium crowded together are called mosaic because their appearance is similar to a mosaic tile. These vessels form a basket around the blocks of abnormal epithelium [Table/Fig-5a,b]. When it is difficult to describe the pattern of the vessels, the term atypical vessels is used. Atypical vessels are terminal vessels that are irregular in size and shape and coarse and such arrangement indicates neoplasia [Table/Fig-6a,b].
(A) Illustration of Punctate capillaries in colposcopic view (B) Punctate vessels in leukoplakia
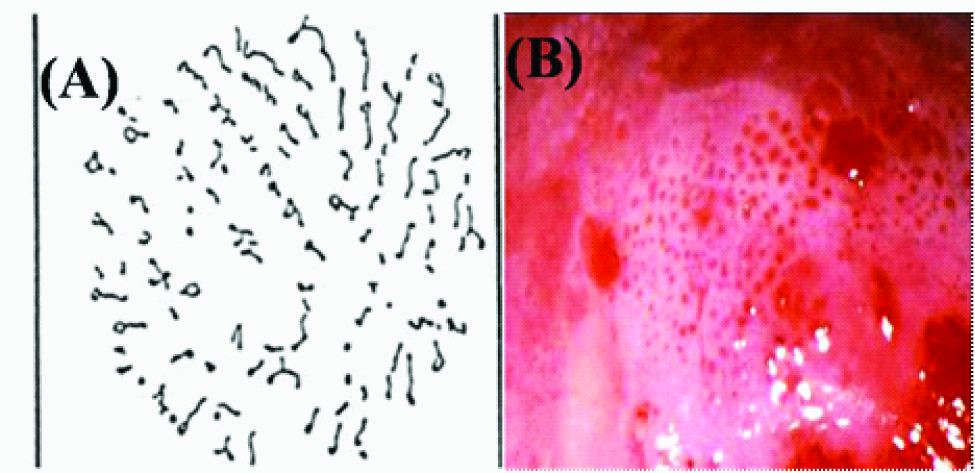
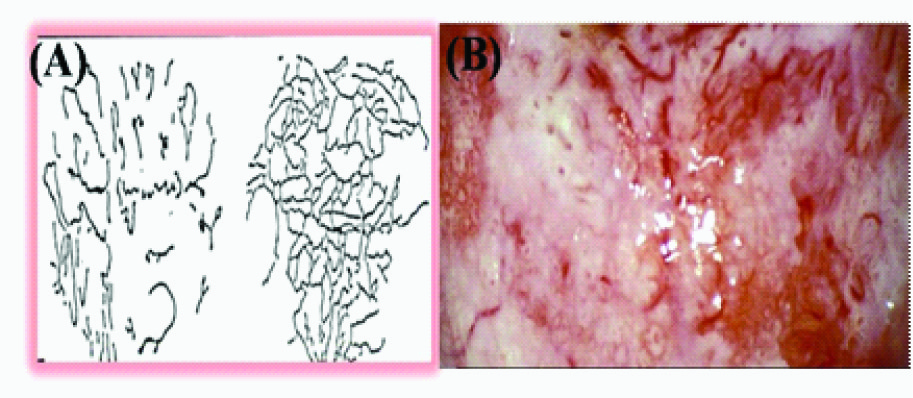
(A)Illustration of mosaic capillaries in colposcopic view (B) Mosaic pattern in leukoplakia pointing towards high grade of dysplasia
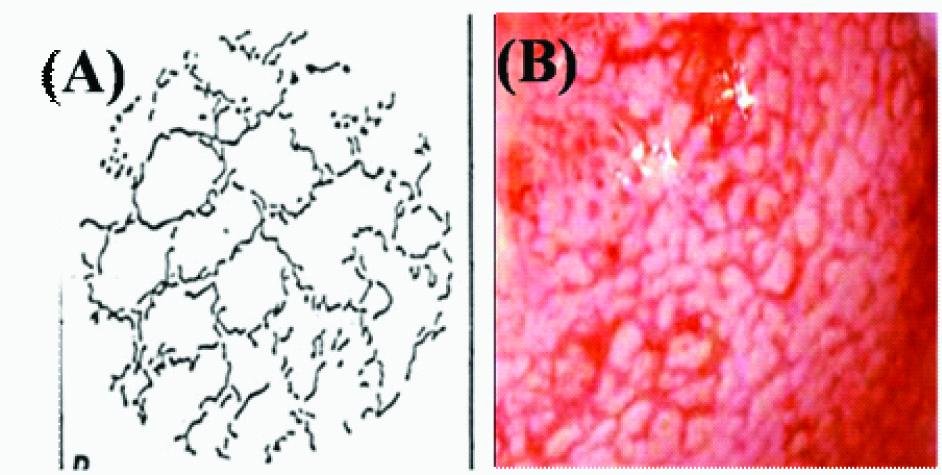
(A)Illustration of atypical capillaries in colposcopic view. (B) Atypical pattern in leukoplakia suggesting of epithelial dysplasia and ongoing malignant transformation
Fine punctuation and mosaicism which are created by narrow vessels and uniform intercapillary distances typify low-grade lesions [17]. A coarse pattern resulting from a wider and more variable vessel diameter and spacing indicates higher grade abnormalities. The mosaic tiles with central punctuation indicate carcinoma in situ. Fracturing of previously intact mosaic and punctuate patterns with the production of predominantly waste thread like vessels is an early colposcopic warning sign of squamous micro invasion or cancer. Thus dilation and proliferation of the resulting punctuate and mosaic patters increase with the degree of neoplastic change.
As the neoplastic growth process proceeds and the need for oxygen and nutrition increases, angiogenesis occurs as a result of tumour and local tissue production of VEGF, PDGF, EGF, and other cytokines, resulting in the proliferation of blood vessels and neovascularization. Atypical vessels may be looped, branched, or reticular. Sharp turns, dilations, and luminal narrowing also characterize these vessels. The surface epithelium may be lost in these areas leading to irregular surface contour and friability. Common to all these vascular patterns is irregular vessel dilatation and intercapillary distances greater than the normal distance of 50-200 μm. With the increasing degree of dysplasia, the distance increases so that the maximum distances may exceed 700 μm [21].
Discussion
Till date, various gynecological methods of examination have been adapted to oral cavity, but with limited success. With Colposcopy, care can be administered more precisely with an enhanced appearance. Colposcope makes it possible to see what a healthy naked eye can’t see. The surface pattern, clarity of demarcation, colour tone, and opacity can be more easily identified by direct oral microscopy than by routine clinical examination. This will aid in the earliest detection of premalignant lesions and conditions such as leukoplakia, lichen planus, lichenoid reaction, and various others [17].
In Sweden, a research conducted by Goron Gnyther, among 35 patients, 29 patients(83%) showed changes in the vascular picture on microscopy, according to the colposcopy criteria. The study concluded that direct oral microscopy of mucosal lesions seems to offer advantages in selecting more representative sites for biopsy than routine clinical examination alone [10]. Ellen H Hopman, in his study stated colposcopy as an effective tool for diagnosing cervical intra-epithelial neoplasia. It was suggested that micro-invasive carcinoma was suspected when mosaic, punctation and acetowhite epithelium was seen with a thick white epithelium that had a clear and elevated margin with an irregular surface contour and raised capillaries [18].
The progression from dysplasia to carcinoma cannot be determined on the basis of the clinical findings. Similarly, such a progression of mucosa cannot be detected because areas of suspected mucosal change may contain foci of varying degrees of dysplasia, reversible or irreversible [10]. Regular follow-up examinations are therefore essential for precancerous lesions, such as inhomogeneous leukoplakia. Hopefully, colposcopy will be used to follow mucosal lesions and detect signs of progression because at present, this seems to be the only way to evaluate vascular changes in the oral mucosa [17].
Compared with other methods, the advantages of colposcopy include high resolution, good magnification, good illumination, good storage capacity, detects lesions at an early stage, painless, non-invasive, with an accuracy of 80-90%. The chief disadvantages are the complexity and cost, and these should be evaluated by further comparative studies.
Conclusion
Colposcopy serves as a promising tool for early diagnosis of lesions and selecting biopsy site due to its high precision, versatility, ease of use and non-invasive technique. Further studies to evaluate its accuracy and efficacy in detecting oral lesions and a comparative evaluation with other methods used are required which could be a step toward continuing to learn and improve the care we offer to our patients. The use of colposcopy should be made in our routine practice, providing better patient care with early diagnosis.
[1]. Nayyar Abhishek Singh, Gayitri HC, Khan Mubeen, Bafna UD, Siddique Ahmed, Colposcopy and carcinoma buccal mucosa: finding significance, a pilot studyInternational Journal of Scientific and Research Publications 2012 2(6):1-7. [Google Scholar]
[2]. Nelson L, Rhodus A, Kerr Ross, Patel Ketan, Oral CancerDCNA 2014 58:315-40. [Google Scholar]
[3]. Axell T, Holmstrum P, Kramer IRH, Pindborg JJ, Shear M, International seminar on oral leukoplakia and associated lesions related to tobacco habits.1983 June 27-30; Lund University, malmo, SwedenCommunity Dent Oral Epid 1984 12:144-54. [Google Scholar]
[4]. Martin IC, Kerawala CJ, Reed M, The application of toluidine blue as a diagnostic adjunct in the detection of epithelial dysplasiaOral Surg Oral Med Oral Pathol Oral RadiolEndod 1998 85:444-46. [Google Scholar]
[5]. Folsom TC, White CR, Bromer L, Oral exfoliative cytology: Review of the literature and report of a 3-year studySurgery 1972 33:61-74. [Google Scholar]
[6]. Epstein JB, Silverman S, Epstein JD, Lonky SA, Bride MA, Analysis of oral lesion biopsies identified and evaluated by visualexamination, chemiluminescence and toluidine blueOral Oncol 2008 44:538-44. [Google Scholar]
[7]. Silverman S. Jr, Gorsky M, Lozada F, Oral leukoplakia nad malignant transformation. A follow up study of 257 patientsCancer 1984 53:563-68. [Google Scholar]
[8]. Karabutul A, Reibel J, Therkildsen MH, Praetorius F, Nielsen HW, Dabelsteen E, Observer variability in the histologic assessment of oral premalignant lesionsJ Oral Pathol Med 1995 24:198-200. [Google Scholar]
[9]. Principles and practices of Colposcopy – Patrick Walker2nd edB. Shakuntala Baliga(Page No-1) [Google Scholar]
[10]. Gynther GW, Rozell B, Heimdahl A, Direct oral microscopy and its value in diagnosing mucosal lesionOral Surg Oral Med Oral Pathol 2000 90:164-70. [Google Scholar]
[11]. Sankaranarayanan R, Wesley R, Thara S, Dhakad N, Chandralekha B, Sebastian P, test characteristics of visual inspection with 4% acetic acid (VIA) and Lugol’s iodine (VILI) in cervical cancer screening in Kerala, IndiaInt J Cancer 2003 106:404-08. [Google Scholar]
[12]. Nagaraju Kamarthi, Prasad Shiva, Ashok L, Diagnostic efficiency of toluidine blue with Lugol’s iodine in oral premalignant and malignant lesionsIndian J Dent Res 2010 21(2):218-23. [Google Scholar]
[13]. Dhakal Hari Prasad, Nesland Jahn M, Forsund Mette, Primary Tumour Vascularity, HIF-1α and VEGF expression in vulvar squamous cell carcinomas: their relationships with clinicopathological characteristics and prognostic impactBMC Cancer 2013 13:506 [Google Scholar]
[14]. Pazouki S, Chisholm DM, Adi MM, Carmichael G, Farquharson M, Ogden GR, The association between tumour progression and vascularity in the oral mucosaJ Pathol 1997 183:39-43. [Google Scholar]
[15]. Chenoy R, Shroff JF, Redmen CW, Gynecology for postgraduares and practitioners. In: Sengupta B, Chattopadhyay S, Dutta DC, editorsChapter 46, Colposcopy 2007 New DelhiChurchill Livingstone:524-34. [Google Scholar]
[16]. Kolstad P, Terminology and definitions. In: Kolstad P, editorAtlas of colposcopy 1982 3rd edLondonChurchill Livingstone:21-31. [Google Scholar]
[17]. Pallagatti Shambulingappa, Sheikh Soheyl, Puri Nidhi, Gupta Deepak, Singh Balwinder, Colposcopy: A new ray in the diagnosis of oral lesions 2011 22(6):810-15. [Google Scholar]
[18]. Hopman EH, Helmerhorst TJ, Colposcopic findings and nomenclatureCME J Gynaecol Oncol 2005 10:11-13. [Google Scholar]
[19]. Dresang LT, Colposcopy: An Evidence-Based UpdateJ Am Board Fam Med 2005 18:383-92. [Google Scholar]
[20]. Alker P, De Palo G, Campion M, Jakob C, Roy M, International terminology of Colposcopy: An updated report from the International Federation for Cervical Pathology and colposcopyObstet Gynecol 2003 101:175-77. [Google Scholar]
[21]. Khan Mubeen, Nayyar Abhishek Singh, Gayitri HC, Bafna UD, Siddique Ahmed, A potential marker of the ongoing process of malignant transformation in leukoplakia patients, removing the veilTumour angiogenesis 2012 1(3):127-34. [Google Scholar]