18F-FDG-PET/CT in a Patient with Suspected Large Vessel Vasculitis
Daniele Penna1, Claudio Testa2, Vincenzo Arena3, Ettore Pelosi4
1 Nuclear Medicine Physician, IRMET S.P.A. Euromedic PET/CT Center, Via Onorato Vigliani 89, Zip Code 10135, Turin, Italy.
2 Radiology Technician, IRMET S.P.A. Euromedic PET/CT Center, Via Onorato Vigliani 89, Zip Code 10135, Turin, Italy.
3 Nuclear Medical Physician, IRMET S.P.A. Euromedic PET/CT Center, Via Onorato Vigliani 89, Zip Code 10135, Turin, Italy.
4 Nuclear Medical Physician, IRMET S.P.A. Euromedic PET/CT Center, Via Onorato Vigliani 89, Zip Code 10135, Turin, Italy.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Daniele Penna, Nuclear Medicine Physician, IRMET S.p.A. Euromedic PET/CT Center, Via Onorato Vigliani 89, Zip Code 10135, Turin Italy. Phone : +390113160158, +393473653560, Fax : +390113160828, E-mail : d.penna@irmet.com
FDG-PET, Giant cells arteritis, Inflammatory processes imaging, Large vessel vasculitis
History
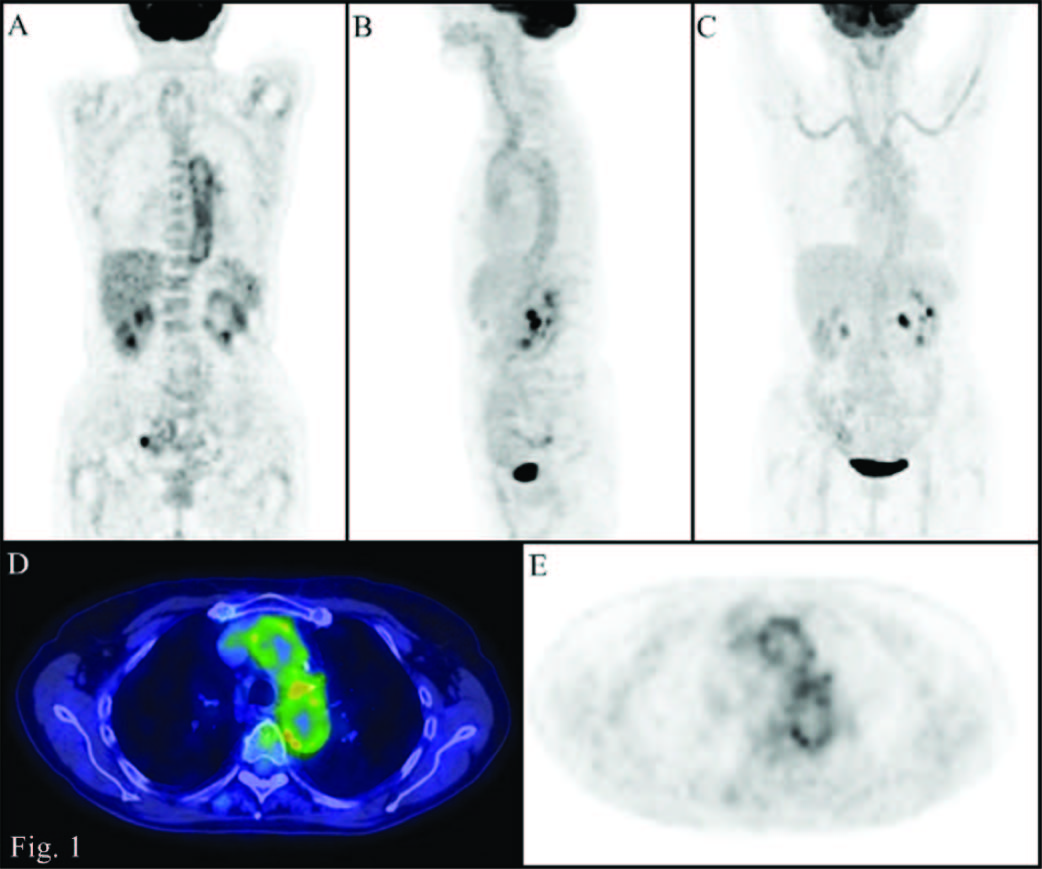
We report the case of a patient, female, 79 years old, suffering from sigmoid diverticulitis, thyroid goiter and hypertension. Following the appearance of claudication, a significant increase of the inflammatory markers was observed (ESR 81 mm/h and CRP 40.6 mg/dL). A Doppler ultrasound of the legs vessels and of the vertebral arteries resulted negative and non indicative for a biopsy. In the clinical suspicion of vasculitis, a total body Positron Emission Tomography/Computed Tomography (PET/CT) was performed. Patient was injected with a dose of 18F-FDG correlated with her weight and the scan was performed after 60 minutes from the injection. PET images were evaluated qualitatively (visual examination) and semiquantitatively (using the maximum Standardized Uptake Value) comparing vessels uptake of radiotracer with the liver background. Both visual and semiquantitative evaluations showed a diffuse abnormal accumulation of 18F-FDG at the level of the aorta and the subclavian, carotid, iliac and femoral arteries [Table/Fig-1]. These PET findings, together with the clinical data, led clinicians to set up a treatment with cortisone (10mg/die in 2 oral somministration) and methotrexate (10mg/week). A second PET scan, performed after 8 months, showed a significant reduction of the vascular abnormal uptakes of radiotracer [Table/Fig-2]. This imaging information, indicative of a partial treatment response, associated with the disappearance of claudication and the reduction of inflammatory markers (ESR 19, CRP 0.5) led physicians to continue therapy. A third PET scan performed after one year from the second, when sympthoms and markers of inflammation were stabilized (ESR 17, CRP 0.7), showed further reduction of the vessels uptake which was less than the liver one [Table/Fig-3].
PET/CT images (A: Coronal PET; B: MIP lateral view; C: MIP front view; D: transaxial Fusion; E: transaxial PET): abnormal accumulation of 18F-FDG at the level of the walls of the great vessels (uptake higher than that of the liver).,
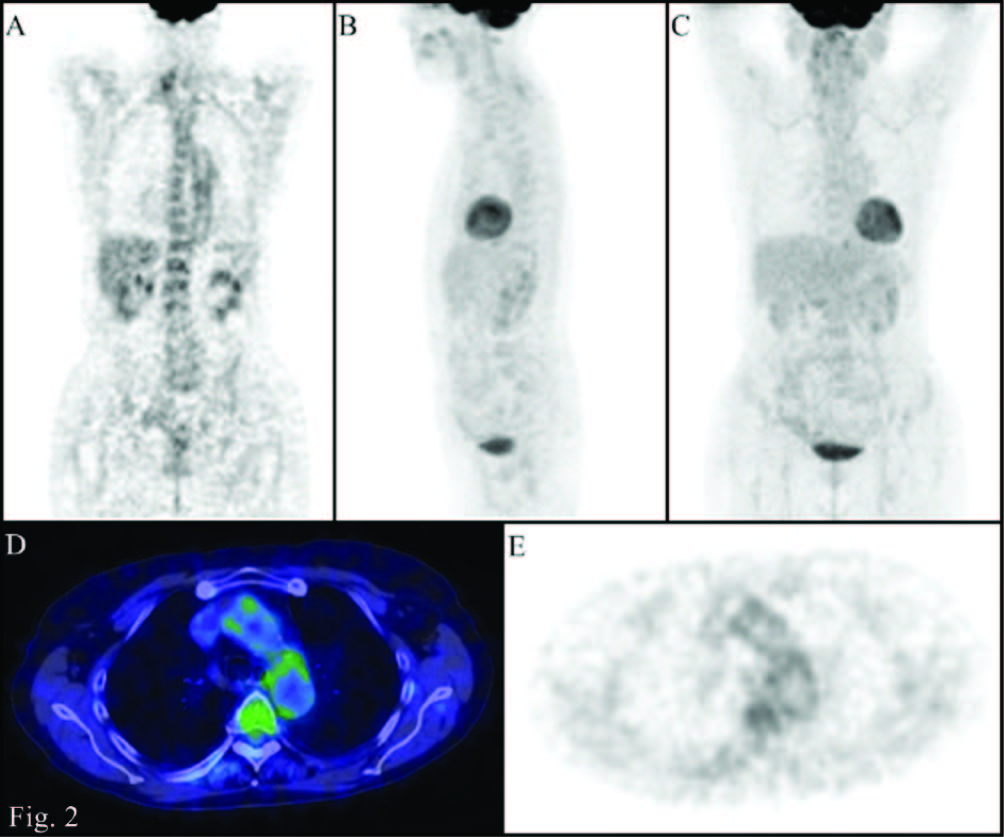
PET/CT images (A: Coronal PET; B: MIP lateral view; C: MIP front view; D: transaxial Fusion; E: transaxial PET): significant reduction in the abnormal accumulation of 18F-FDG at the level of the walls of the great vessels during the treatment (uptake similar to that of the liver)
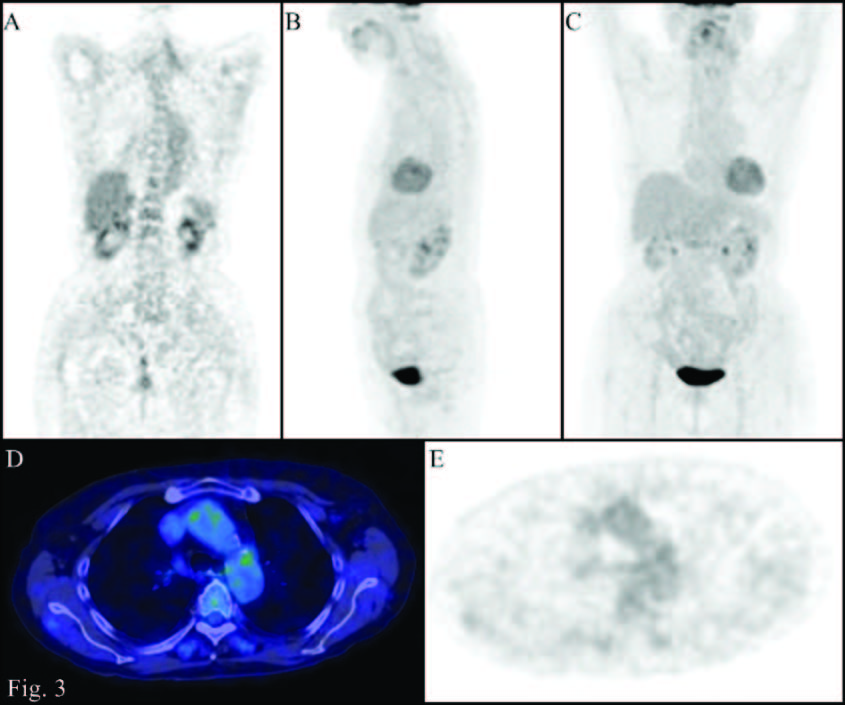
PET/CT images (A: Coronal PET; B: MIP lateral view; C: MIP front view; D: transaxial Fusion; E: transaxial PET): after further treatment, further reduction of the accumulation of 18F-FDG at the level of the walls of the great vessels (uptake minor to that of the liver)
Discussion
Vasculitis refers to a heterogeneous group of rare diseases that have in common an inflammatory condition of the arterial vessel wall [1, 2]. Giant Cells Arteritis (GCA) represent the most common form of vasculitis in Western countries and are characterized by the frequent involvement of the thoracic aorta and its branches [3]. The diagnostic workup in the study of vasculitis can be complex because of the multiple clinical aspects: the possible association with rheumatic polymialgia, the presence of some non-specific symptoms, the correlation with levels of serological markers, such as erythtrocyte sedimentation rate (ESR), C-reactive protein (CRP), antinuclear antibodies (ANA) and perinuclear antineutrophil cytoplasmic antibodies (pANCA) [2, 4]. Furthermore the temporal artery biopsy, which is considered the gold standard, even if negative would not completely exclude the presence of disease [3, 5]. CT, MRI and ultrasound are the imaging modalities commonly used as a support in the workup of vasculitis [2, 6]. Instead only recent studies are showing a potential role of PET/CT in the diagnosis and in the evaluation of treatment response [3, 4].
Therefore the difficulties in the diagnosis of patients with vasculitis depend on the heterogeneity of this group of diseases. Specifically about the GCA, that involves large and medium vessels, the criteria from the American College of Rheumatology (ACR) provide the confirmation of the disease when at least three of the following five parameters are present: age older than 50 years, recent localised headache, temporal artery pulse attenuation, ESR> 50 mm/h and positive arterial biopsy. These criteria can reach a diagnosis with a sensitivity of 94% and a specificity of 91%, however they do not include the possible support of imaging findings [2, 7]. CT, MRI and Ultrasonography can be used in the evaluation of the wall thickening and the vessel wall edema that are usually associated to the disease [2, 6, 8]. Many studies have recently been performed in order to evaluate the potential use of PET in the investigation of these diseases on the basis of the high sensitivity of this tool to identify tissues involved by inflammatory processes [3, 4]. The accumulation of 18F-FDG is related to the presence of inflammatory cells, such as giant cells and macrophages. These cells in vasculitis play the role of mediators in the neoangiogenesis process of vasa vasorum [9]. The limitations of PET images is instead related to the spatial resolution that in current tomographs is about 5 mm and therefore allows to evaluate the vessels of great size, excluding the intracranial compartment [2, 10]. Some criteria for interpretation of PET images in vasculitis have been defined in literature using a semiquantitative scale which compares the accumulation of radiotracer at the level of the great vessels walls to that of the generally homogeneus liver background [3,4]. In our study PET images were first assessed qualitatively by three nuclear physicians who have identified the presence of an abnormal increase of fixation of the radiotracer at the level of the great vessels at the first PET scan performed and a progressive reduction of this finding in the following two PET scans. Moreover, on the basis of some interpretation criteria from literature, we evaluated the ratio (R) between the uptake of the vessels and that one of the liver, considering the finding “positive” in the presence of a value greater or equal to 1 and “negative” if less than 1 [3,4]. In the case reported we observed a significantly positive ratio at the first PET scan (R = 1.33), that allowed physicians to reach a clinical diagnosis of great vessels vasculitis, even if that was in discordance with the ACR criteria (only two parameters were present ESR value and age of the patient) and in the absence of biopsy that, however, in the study of these diseases has a high percentage of false negatives that can reach 40% [3,5]. This diagnosis was also confirmed by two successive PET examinations performed during the treatment, which showed a significant reduction of abnormal vascular accumulations (R=1 at second PET scan, R=0.77 at third PET scan), associated with clinical improvement and a reduction in inflammatory markers. Therefore the introduction of the PET in this study has been useful in the diagnosis of vasculitis and also has shown a high ability of this tool in the evaluation of the treatment response, as already highlighted in other studies [2]. Further clinical trials are necessary in order to confirm the use of PET scan in this field, together with cost-effective studies.
[1]. Hiratzka LF, Bakris GL, Beckman JA, 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular MedicineCirculation 2010 121(13):e266-e369. [Google Scholar]
[2]. Litmanovich DE, Yıldırım A, Bankier AA, Insights into imaging of aortitisInsights Imaging. 2012 3(6):545-60. [Google Scholar]
[3]. Besson FL, Parienti JJ, Bienvenu B, Diagnostic performance of 18F-fluorodeoxyglucose positron emission tomography in giant cell arteritis: a systematic review and meta-analysisEur J Nucl Med Mol Imaging. 2011 38(9):1764-72. [Google Scholar]
[4]. Salvarani C, Pipitone N, Versari A, Positron emission tomography (PET): evaluation of chronic periaortitisArthritis Rheum 2005 15:53(2):298-303. [Google Scholar]
[5]. Gonzalez-Gay MA, Garcia-Porrua C, Llorca J, Biopsy-negative giant cell arteritis: clinical spectrum and predictive factors for positive temporal artery biopsySemin Arthritis Rheum. 2001 30(4):249-56. [Google Scholar]
[6]. Pipitone N, Versari A, Salvarani C, Role of imaging studies in the diagnosis and follow-up of large-vessel vasculitis: an updateRheumatology (Oxford). 2008 47(4):403-08. [Google Scholar]
[7]. Hunder GG, Bloch DA, Michel BA, The American College of Rheumatology 1990 criteria for the classification of giant cell arteritisArthritis Rheum 1990 33(8):1122-28. [Google Scholar]
[8]. Bongartz T, Matteson EL, Large-vessel involvement in giant cell arteritisCurr Opin Rheumatol. 2006 18(1):10-17. [Google Scholar]
[9]. Kaiser M, Younge B, Björnsson J, Formation of new vasa vasorum in vasculitis. Production of angiogenic cytokines by multinucleated giant cellsAm J Pathol. 1999 155(3):765-74. [Google Scholar]
[10]. Walter MA, [(18)F]fluorodeoxyglucose PET in large vessel vasculitisRadiol Clin N Am 2007 45(4):735-744.:viii [Google Scholar]