Case Report
A 65-year-old male, farmer by profession presented with complaints of gradually progressive painful swelling in the right chest wall region for 10 months. Swelling was first noticed as a small lump of size 2x2 cm which gradually progressed to the size of 15x12 cm [Table/Fig-1a]. There was no history of fever or nipple discharge.
A 15x12 cm right sided chest wall mass (a) which was filled with 500 ml blood clots on cut section (b) and (c) showed a small round cell tumour on histopathology (Hematoxylin and Eosin 10x)
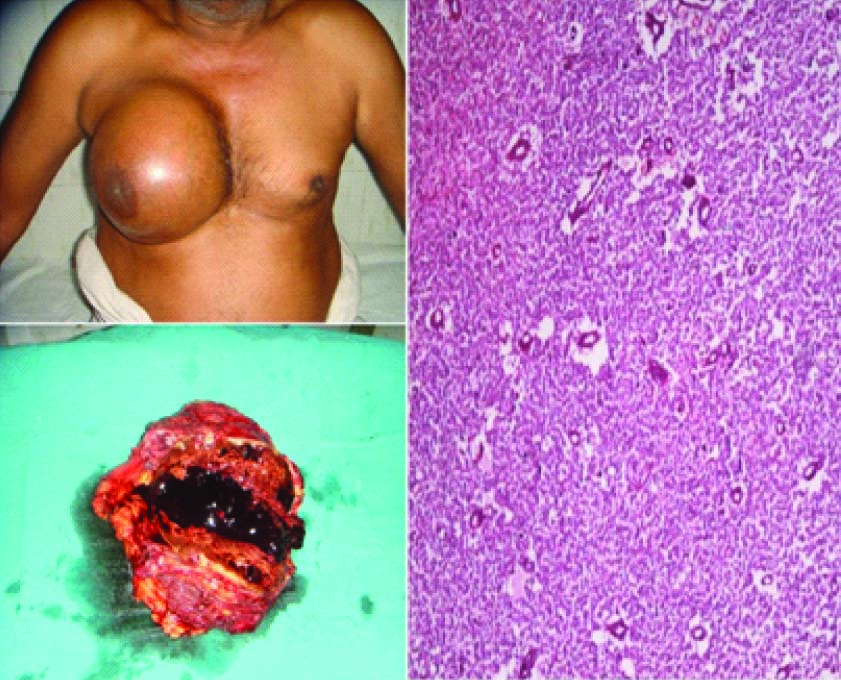
The patient was an average built man with a large swelling of about 15x12 cm involving whole of the right breast and chest wall area. There was no local rise in temperature and the swelling was fluctuant in consistency. The skin above the swelling was stretched and could be lifted separately from the lump. There was no ulceration and the nipple areola complex was normal. There was no axillary lymphadenopathy and the contralateral breast was normal.
The patient had undergone fine needle aspiration cytology elsewhere which was suggestive of a malignant tumour. The Contrast Enhanced Computed Tomography (CECT) was suggestive of malignant right breast mass with areas of necrosis.
The patient underwent radical resection of the tumour. The pectoralis major muscle was infiltrated by the tumour and it was excised with the tumour. On opening the tumour mass, 500 ml blood clots were evacuated [Table/Fig-1b].
On cut section, the tumour was a 13x12 cm globular soft tissue mass with large areas of haemorrhage and necrosis. No papillary excrescences were noted on the wall of the tumour. It was grey brown in colour, fleshy in consistency with several blood clots. The attatched skeletal muscle appeared to be infiltrated by the tumour.
Histopathologic examination of the tumour revealed a small round cell tumour with focal peritheliomatous arrangement and vague pseudorosettes [Table/Fig-1c]. No true rosettes were found. No features of rhabdomyoblastic differentiation were noted. Several slit like and angulated blood vessels and large areas of haemorrhage were noted. The tumour had infiltrative margins and invaded the surrounding fibroadipose tissue and skeletal muscles. The tumour cells had vesicular chromatin and scant to absent cytoplasm. Occasional mitotic figures were noted with few areas showing coagulative necrosis. The tumour cells showed frequent indentations and small nucleoli. Periodic acid Schiff (PAS) positive and diastase sensitive substance (glycogen) could be demonstrated in the tumour cells. Based on these histomorphologic features, the differential diagnosis included lymphoblastic lymphoma, primitive neuroectodermal tumour/extraskeletal Ewings sarcoma, small cell carcinoma, and rhabdomyosarcoma. The tumour cells were immunopositive for vimentin and focally positive for cytokeratin and negative for leucocyte common antigen and desmin. Due to logistical and technical issues an extended panel could not be put up and a diagnosis of malignant small round cell tumour was rendered. The patient was advised to get further immunohistochemistry done on the blocks. However, the patient was lost to follow up.
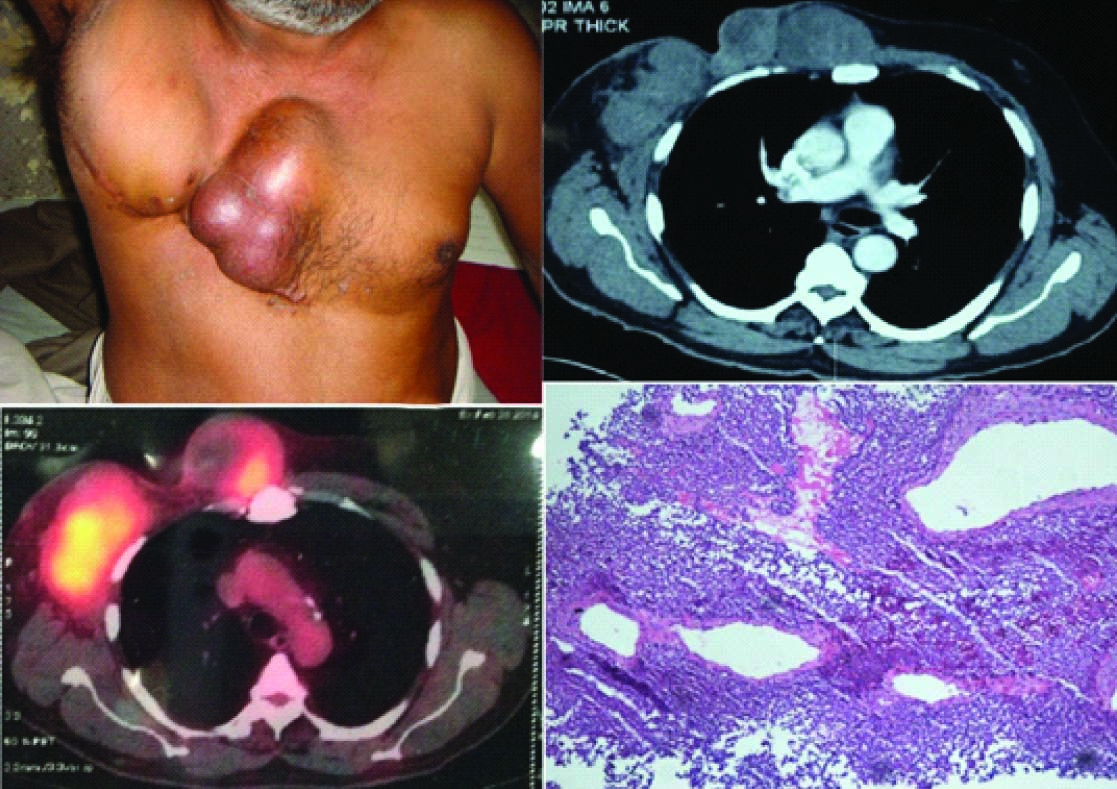
The patient came back after one year with complaints of multiple swellings in the right anterior chest wall for four months, initially pea size which gradually progressed to the size of an orange. On examination the patient was having three swellings each of size 8x6cm,6x5cm, and 5x5 cm with no local rise of temperature [Table/Fig-2a]. The swellings were firm to cystic in consistency and were fixed to the chest wall. There was no ulceration of the overlying skin. The contrast enhanced Computed Tomography and FDG PET (Positron Emission Tomography) both confirmed recurrence at the local site [Table/Fig 2b,2c]. A tru cut biopsy was performed which showed histological features similar to the resection specimen [Table/Fig-2d].
(a) Tumour recurrence with three swellings fixed to the chest wall (b) Enlarged enhancing masses in anterior chest wall( right hemithorax) with loss of fat planes with intercostal muscles and scalloping of underlying ribs on Contrast Enhanced Computed Tomography (c) Hypermetabolic soft tissue density mass lesions along presternal region, right hemithorax and right axilla inseparable from underlying chest wall muscles were noted on FDG PET(Positron Emission Tomography) (d) The trucut biopsy showed a small round cell tumour with peritheliomatous pattern similar to the resection specimen (Hematoxylin and Eosin 10x)
An immunohistochemistry panel comprising of following antibodies was applied to the retrieved blocks of resection specimen- mic-2(CD99),Fli-1(Friend Leukemia Integration transcription factor-1),NSE (Neuron Specific Enolase),CG (Chromogranin),LCA(Leucocyte Common Antigen),TdT(Terminal deoxynucleotidyl Transferase), desmin, CK(pan Cytokeratin) and vimentin [Table/Fig-3a-f].
On immunohistochemistry the tumour showed membranous staining for mic-2 (a) and neuron specific enolase (c) and nuclear staining for Friend Leukemia Integration transcription factor 1(FLI1) (b). It was focally immunopositive for pan cytokeratin (f) and was negative for desmin (d) and Terminal deoxynucleotidyl transferase (TdT)(e)

Based on the pattern of immunostains, a diagnosis of Primitive Neuroectodermal Tumour/Extraskeletal Ewings Sarcoma was made [Table/Fig-3,4]. The status of t (11,22) of the tumour could not be ascertained .
A simplified scheme showing the immunostaining pattern of the tumour compared with the usual pattern of other differential diagnoses
| PNET/ES | Small cell Carcinoma | Lymphoblastic Lymphoma | Rhabdo myosarcoma | Our case |
|---|
| CD99 | +/-(rare) | - | +/- | -/+(rare) | + |
| FLI1 | +/-(rare) | - | +/- | - | + |
| NSE | +/- | + | - | - | + |
| CG | +/- | + | - | - | + |
| LCA | - | - | +/- | - | - |
| TdT | - | - | + | - | - |
| Desmin | - | - | - | + | - |
| Pan CK | +/- | Nuclear dot + | - | - | + |
| Vimentin | + | -/+ | - | + | + |
PNET-Primitive neuroectodermal tumour, ES-Ewings Sarcoma, FLI1-Friend Leukemia Integration Transcription Factor 1,NSE-Neuron Specific Enolase, CG-Chromogranin, LCA-Leucocyte Common Antigen, TdT- Terminal deoxynucleotidyl transferase, Pan CK-Pan Cytokeratin
The patient was given chemotherapy at a specialised oncology center for the tumour and was showing good response to the therapy at the time of writing of the case report.
Discussion
Askin FB et al., first described in 1979, the tumour now named after him, in 20 children and adolescents (average age 14.5 y) [1]. The Askin tumour is now simply regarded as an example of Primitive neuroectodermal tumour /Ewings Sarcoma occurring in the chest wall. Primitive neuroectodermal tumours are small round cell tumours of neural crest origin first described in central nervous system but more recently in the peripheral nervous system [2]. Peripherally located PNETs are members of the Ewings Sarcoma family of tumours [3].
The pathogenetic unity of Ewings Sarcoma of bone and Primitive neuroectodermal tumour of soft tissue is supported by the fact that both harbour the same 11;22 chromosomal translocation [4]. It had been generally accepted, that the bone tumours are more undifferentiated and the soft tissue counterparts show better evidence of neuroectodermal differentiation [5]. However neural differentiation has been induced in conventional Ewings Sarcoma by agents such as retinoic acid [6].
Microscopically the typical cases of ES/PNET show sheets of small round cells with fibrous strands dividing them into irregular masses. The cell outlines are indistinct resulting in syncytial appearance.
The nuclei have frequent indentations ,small nucleoli and variable mitotic activity. The cells of ES/PNET usually contain large amounts of glycogen which can be demonstrated by Periodic Acid Schiff (PAS) stain with diastase control. However, it is not specific for this tumour [7].
CD99 (mic- 2) is cell membrane protein which is consistently expressed by cells of ES/PNET. In view of the overlap of expression of different immunomarkers amongst the different small round cell tumours [Table/Fig-4] a panel of antibodies is essential to reach a diagnosis [8] .
About 95% of cases of ES/PNET show the reciprocal translocation t(11,22)(q24;q12) or t(11,22)(q22;q12) which results in the fusion of the EWS(Ewings Sarcoma) gene at 22q12 with FLI1 or ERG gene respectively[4] .There are no phenotypic differences between the cases associated with EWS-FLI1 and those associated with EWS-ERG [9]. The diagnostic utility of this gene fusion remains a debatable issue. This gene fusion has been recorded in other tumours also [10].
A particularly challenging differential diagnosis on histomorphology is lymphoblastic lymphoma. Even on immunohistochemistry there is some overlap since CD99 and FLI1 has been reported in rare cases of lymphoblastic lymphoma. Contrary to the general perception even Leucocyte Common Antigen fails to solve the puzzle. Only TdT (Terminal deoxynucleotidyl Transferase) can differentiate between the two [11].
The metastatic spread of the tumour to lungs, pleura, other bones, central nervous system and regional lymph nodes has been recorded.
The role of FDG PET is well established in postoperative work up and evaluation of response to chemotherapy in childhood sarcomas like PNET. However, for diagnostic purposes it does not have much role. High standard uptake values (SUVs) are expected since PNETs are high grade malignancies. But, the reported SUVs are much lower which may be due to lower expression of glucose transporters or variability in the expression of glucose transporters between primary and metastatic sites [12].
The imaging features of Askins tumour are not specific. MRI is more sensitive in evaluating local invasion by providing true multiplanar images and better soft tissue differentiation but CT (Computed Tomography) is considered better for detecting lung and remote metastasis and bony details [13].
Few case reports of Askin tumour in elderly patients have appeared in literature [13,14] however the occurrence is extremely rare.
The treatment of ES/PNET has improved dramatically. The five year survival rate was less than 10% in the past but has now improved to 75% with the use of multimodal therapy which includes surgery, high dose irradiation and multidrug chemotherapy [15]. The chemotherapy regimens that have been used include VAC( Vincristine, Actinomycin D, cyclophosphamide), VACA (VAC+ Adriamycin) and VAC alternating IE (Ifosfamide and etoposide) [16]. The adverse prognostic factors include advanced age, metastatic disease, extraosseous primary tumour and recurrence [17].
In our patient advanced age, local recurrence and extraosseous primary tumour were poor prognostic factors .However there was no evidence of metastatic tumour on FDG PET and Computed Tomography which were done at the time of recurrence.
Conclusion
This case highlights the importance of keeping Ewings Sarcoma/Primitive neuroectodermal tumour in the differential diagnosis of chest wall masses even in elderly. An accurate early diagnosis can improve the patient outcome with the use of multimodal chemotherapy.
PNET-Primitive neuroectodermal tumour, ES-Ewings Sarcoma, FLI1-Friend Leukemia Integration Transcription Factor 1,NSE-Neuron Specific Enolase, CG-Chromogranin, LCA-Leucocyte Common Antigen, TdT- Terminal deoxynucleotidyl transferase, Pan CK-Pan Cytokeratin
[1]. Askin FB, Rosai J, Sibley RK, Dehner LP, McAlister WH, Malignant small cell tumour of the thoracopulmonary region in childhood: a distinctive clinicopathologic entity of uncertain histogenesisCancer 1979 43(6):2438-51. [Google Scholar]
[2]. Parham DM, Roloson GJ, Feely M, Green DM, Bridge JA, Beckwith JB, Primary malignant neuroepithelial tumours of the kidney: a clinicopathologic analysis of 146 adult and pediatric cases from the National Wilms’ Tumour Study Group Pathology CenterAm J Surg Pathol 2001 25(2):133-46. [Google Scholar]
[3]. Dogra PN, Goel A, Kumar R, Das PK, Gupta SD, Extraosseous Ewing’s sarcoma of the kidneyUrol Int 2002 69(2):150-52. [Google Scholar]
[4]. De Alava E, Pardo J, Ewing tumour: tumour biology and clinical applicationsInt J Surg Pathol 2001 9(1):7-17. [Google Scholar]
[5]. Shishikura A, Ushigome S, Shimoda T, Primitive neuroectodermal tumours of bone and soft tissue: histological subclassification and clinicopathologic correlations Acta Pathol Jpn 1993 43(4):176-86. [Google Scholar]
[6]. Noguera R, Triche TJ, Navarro S, Tsokos M, Llombart-Bosch A, Dynamic model of differentiation in Ewing’s sarcoma cells. Comparative analysis of morphologic, immunocytochemical, and oncogene expression parametersLab Invest 1992 66(2):143-51. [Google Scholar]
[7]. Llombart-Bosch A, Machado I, Navarro S, Bertoni F, Bacchini P, Alberghini M, Histological heterogeneity of Ewing’s sarcoma /PNET: an immunohistochemical analysis of 415 genetically confirmed cases with clinical supportVirchows Arch 2009 455:397-411. [Google Scholar]
[8]. Meis-Kindblom JM, Stenman G, Kindblom LG, Differential diagnosis of small round cell tumoursSemin Diagn Pathol 1996 13:213-41. [Google Scholar]
[9]. Ginsberg JP, de Alava E, Ladanyi M, Wexler LH, Kovar H, Paulussen M, EWS-FLI1 and EWS-ERG gene fusions are associated with similar clinical phenotypes in Ewing’s sarcomaJ Clin Oncol 1999 17(6):1809-14. [Google Scholar]
[10]. Thorner P, Squire J, Chilton-MacNeil S, Marrano P, Bayani J, Malkin D, Is the EWS/FLI-1 fusion transcript specific for Ewing sarcoma and peripheral primitive neuroectodermal tumour? A report of four cases showing this transcript in a wider range of tumour typesAm J Pathol 1996 148(4):1125-38. [Google Scholar]
[11]. Lucas DR, Bentley G, Dan ME, Tabaczka P, Poulik JM, Mott MP, Ewing sarcoma vs lymphoblastic lymphoma. A comparative immunohistochemical study Am J Clin Pathol 2001 115(1):11-7. [Google Scholar]
[12]. Györke T, Zajic T, Lange A, Schafer O, Moser E, Mako E, Impact of FDG PET for staging of Ewing sarcomas and primitive neuroectodermal tumoursNuclear Medicine Communications 2006 27(1):17-24. [Google Scholar]
[13]. Ravaux S, Bousqoet JC, Vancina S, Askin’s tumour in a 67-year-old man with cancer of the prostate. X-ray computed tomography aspectsJournal de Radiologie 1990 71(3):233-36. [Google Scholar]
[14]. Sikri V, Sobti S, Askin tumour: a rare thoracopulmonary tumour in adultsIndian J Chest Dis Allied Sci 2013 55(4):233-35. [Google Scholar]
[15]. Razek A, Perez CA, Tefft M, Nesbit M, Vietti T, Burgert EO Jr, Intergroup Ewing’s Sarcoma Study: local control related to radiation dose, volume, and site of primary lesion in Ewing’s sarcomaCancer 1980 46(3):516-21. [Google Scholar]
[16]. Venkitaraman R, George MK, Ramanan SG, Sagar TLC, A singular institution experience of combined modality management of extraskeletal Ewing’s sarcomaWorld J Surg Oncol 2007 11:5-13. [Google Scholar]
[17]. Baldini EH, Demetri GD, Fletcher CDM, Foran J, Marcus KC, Singer S, Adults with ES/PNET: adverse effect of older age and primary extraosseous disease on outcomeAnn Surg 1999 230:79-86. [Google Scholar]