Ameloblastoma is an epithelial odontogenic tumour of uncertain histogenesis and peculiar clinical behaviour and course [1,2].
The possible sources of cells forming Ameloblastoma have been summarized by Hinds et al., and Gorlin et al., [2,3], which include the stratified squamous epithelium of oral cavity as one of the sources. Recombinant experiments and studies by Mina [4,5], have shown that there exists a narrow window period in the early embryogenesis, during which oral ectoderm is the source of inductive signals for tooth development and for establishing odontogenic competence in oral ectomesenchyme [6]. The involvement of neural crest cells in odontogenesis is well recognised during which they may impart few of their characteristics to the oral epithelium through signal transductions [6–8].
This study is based on the surmise that the basal cells of the oral epithelium, particularly in the retromolar area (last region to witness odontogenic epithelial-ectomesenchymal signalling), as well as the odontogenic epithelium of Ameloblastoma may possess and continue to express neuro-ectodermal determinants years after completion of odontogenesis [9,10].
By the virtue of being the initiator of tooth development and also possessing few of the ectomesenchymal characters (imparted by neural crest cells), the basal cells may possess a hidden potential to proliferate, when ambient conditions are met, thus becoming a resourceful candidate for giving rise to Ameloblastoma.
Additionally, CK 19 (cytokeratin 19), a proposed novel marker for odontogenic epithelium was used to ensure that the antigenicity was preserved in the tissues [15–18].
Materials and Methods
Paraffin embedded, tissue blocks of 20 cases of Ameloblastoma and 10 cases of retromolar tissue were collected from varied sources including Oxford Institute of Dental Science and Research centre, Bangalore, India. The samples of Ameloblastoma included its histopathological variants like follicular, plexiform, granular, acanthomatous and Ameloblastoma with extensive cystic changes. Tissue sections of 4μm thickness were obtained from all the paraffin blocks and subjected to immunohistochemical staining using antibody kits for the known neuroectodermal markers- NSE, Synaptophysin and CD99 (primary antibodies were obtained from Biogenex and secondary kit from Novocastra). The stained sections were then analysed for staining characteristics. In addition, the tissues were also stained with anti-CK19 antibody to avoid biased results.
The sections were assessed for positive and negative staining. In the normal retromolar mucosa the staining was considered positive only if there was staining in the basal layer and the two layers above it, irrespective of whether the staining was obtained in the superficial layers or not.
To further substantiate the study, the results were statistically analysed using Fischer’s Exact test.
Results
All the specimens, both retromolar tissues and Ameloblastomas, showed diffuse or patchy positivity for CK-19 in the epithelium, establishing their immune reactivity.
Expression of Synaptophysin, NSE and CD99 in Ameloblastoma [Table/Fig-1,2,3,45]:
Expression of synaptophysin, nse and cd99 in ameloblastoma
| Antibody | Positive cases (Total cases-20) | Negative cases |
|---|
| Synaptophysin | 20 | Nil |
| NSE | 20 | Nil |
| CD99 | 06 | 14 |
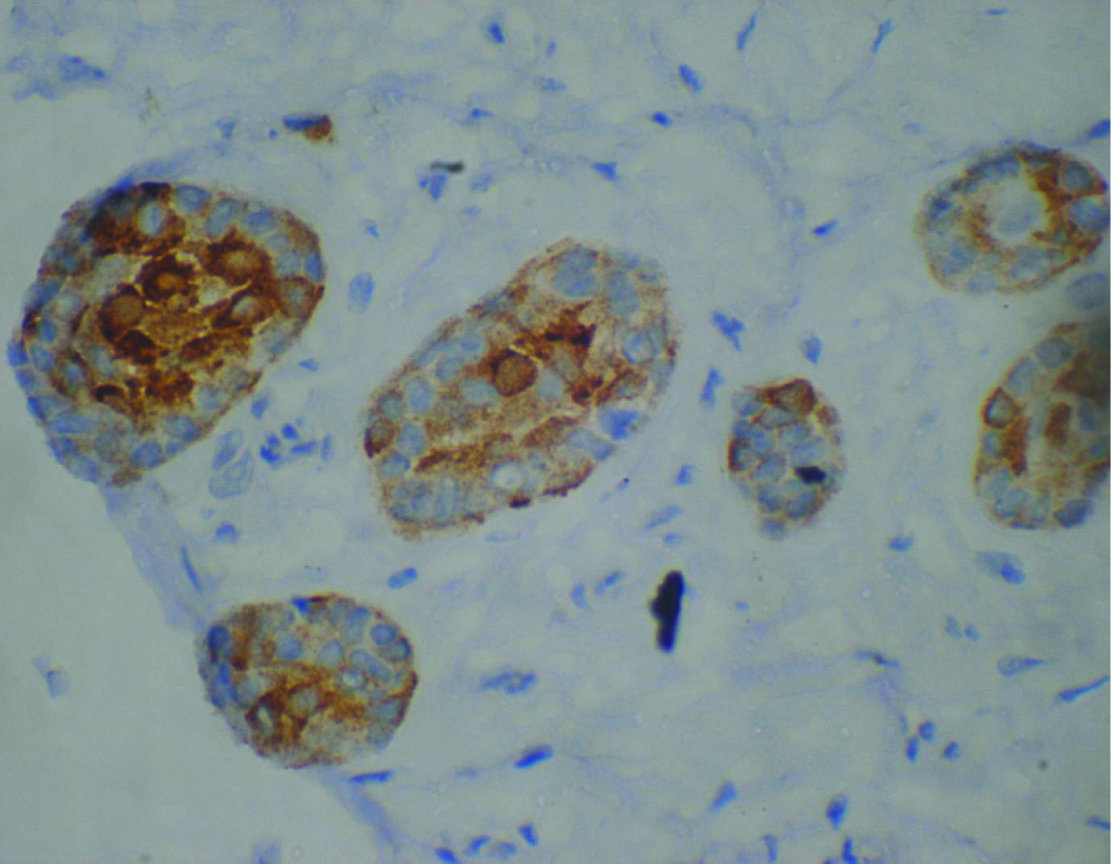
Intense ck-19 staining of the ameloblastic follicles 20x

Synaptophysin expression in ameloblastic follicles 20X

Diffuse positivity for nse in ameloblastic follicles –20x

CD99 staining in ameloblastic follicles 10x

All the 20 cases of Ameloblastoma studied [Table/Fig-4], showed a positive reaction to Synaptophysin [Table/Fig-3] and NSE [Table/Fig-4]. Of these, diffuse positivity was observed in all the 20 cases for NSE [Table/Fig-4] and in 16 cases for Synaptophysin [Table/Fig-3] as shown. Four of the cases showed patchy positivity for Synaptophysin. CD99 [Table/Fig-5] staining was observed in only six cases, of which, two were diffusely positive and the staining was faint to moderate, membrane or granular cytoplasmic.
Expression of Synaptophysin, NSE and CD99 in Normal Retromolar mucosa [Table/Fig-6,7,8,9,10]:
Expression of synaptophysin, nse and cd99 in normal retromolar mucosa
| Antibody | Positive cases (Total cases-10) | Negative cases |
|---|
| Synaptophysin | 08 | 02 |
| NSE | 10 | Nil |
| CD99 | 06 | 04 |
Ck-19 staining in the basal cells of NRM - 40x
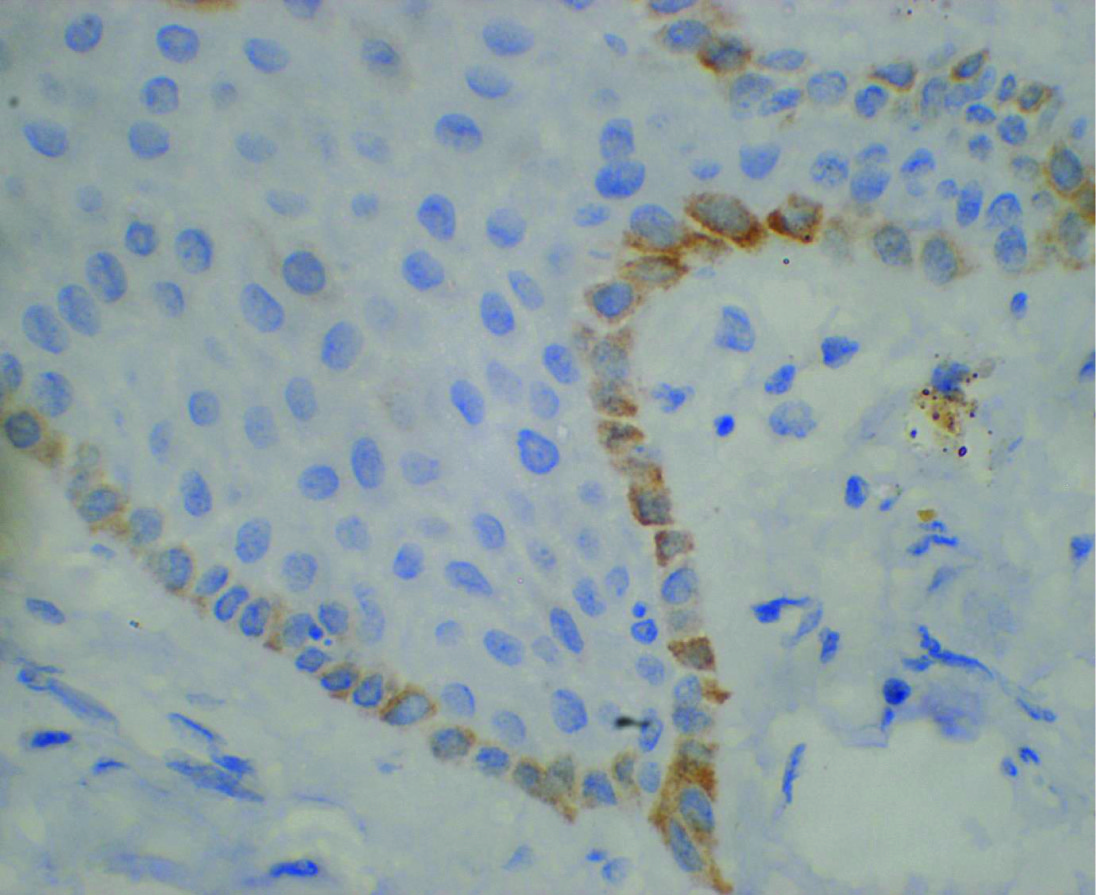
Diffuse positivity of NSE in NRM

Faint positivity to synaptophysin in NRM 20X
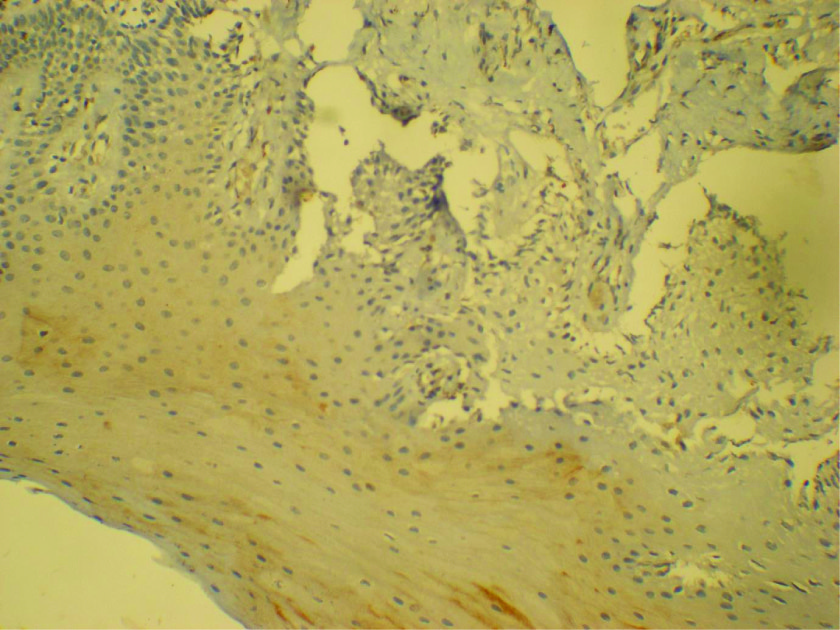
Faint CD99 staining in the basal cells of NRM - 20X

Based on the criteria followed for assessing the expression in the normal mucosa, it was seen that a positive staining was obtained in all the 10 cases for NSE [Table/Fig-8], in eight cases for Synaptophysin [Table/Fig-9] and in six cases for CD99 [Table/Fig-10]. The staining was stronger,diffuse and extended to the superficial layers in case of NSE, and was specifically restricted to the basal layers in case of CD99 [Table/Fig-10].
Comparison of the expression of the markers in Normal Retromolar mucosa and Ameloblastoma:
The expressions of individual markers in Ameloblastoma were correlated with NRM (Normal Retromolar mucosa). There was no significant statistical difference observed in the expression of the markers between the two tissues (p>0.05) as shown in [Table/Fig-11].
Comparison of the expression of the markers in normal retromolar mucosa and ameloblastoma
| Antibody | Normal mucosa | Ameloblastoma | P-value |
|---|
| CK-19 | 10 | 20 | 1.00 |
| Synaptophysin | 8 | 20 | 0.103 |
| NSE | 10 | 20 | 1.00 |
| CD99 | 6 | 6 | 0.139 |
Discussion
The concept of pathological lesions arising in the body taking its origin from normal tissue counterparts is never questioned and is well accepted. To understand any lesion thoroughly, predict its prognosis and design an appropriate treatment plan, knowledge about its origin is mandatory.
Ameloblastoma, a rare but significant odontogenic tumour, is justly considered the most inexplicable of the odontogenic tumours because of its aggressive behaviour and manifold histopathological presentations [1,2,19,20]. Equally intriguingly contradictory and incongruent is its origin, a matter of great speculation and research. It has been suggested to arise directly from the enamel organ of the developing tooth, the remnants of the odontogenic epithelium, the lining of odontogenic cysts or the basal layer of the oral mucosa [2,3,21–24].
Odontogenesis is the culmination of numerous sequential genetic and epigenetic events [5,6]. Aberrant proliferations on account of disturbances in control mechanisms of these ordered events can result in an array of odontogenic tumours, including Ameloblastoma.
Though the initial odontogenic signal rests in oral epithelium, involvement and interaction between the two parent tissues of the dentition, the oral epithelium and the ectomesenchyme is a necessary event during odontogenesis [6–8]. During this process, the ectomesenchyme has a profound effect on the overlying epithelium and presumably transfers some of its characteristic features or traits to it. With the completion of intended function, change in the signalling patterns alters the nature of the tissues and their odontogenic capacity becomes redundant. Whether these effects are lost with time or remain latent in the cells of the epithelium, especially the basal layer, since these are the ones in intimate contact with the ectomesenchyme, is food for thought.
A point open to conjecture is that an abnormal triggering of odontogenic signalling pattern in the tissues at any point of time after cessation of odontogenesis, could initiate a similar process leading to proliferation of oral epithelium. It is then safe to assume that the oral ectoderm, which has interacted closely in process of odontogenesis with the ectomesenchyme, which is essentially neuroectoderm, may have acquired some neuroectodermal characteristics.
And if indeed, there has been such an acquisition in the odontogenic period, will any aberrant proliferation of this epithelium result in odontogenesis all over again? Unlikely, since the surrounding environment is no longer the same and the ectomesenchyme has been replaced in the region by a mature connective tissue and may not possess the necessary potential to promote odontogenesis. There is no longer formation of the tooth bud and instead the epithelium would continue to proliferate abnormally. This might result in the formation of Ameloblastoma.
Studying the expression of neuroectodermal markers in the representative Oral epithelium and the Amelobastoma might throw light on such an assumed potential thus providing an insight to the tumorigenesis.
A previous study done on 32 cases of Ameloblastoma, gave the first evidence of such a link between the Neuroectoderm and the Ameloblastoma, when it was seen that the Ameloblastoma possessed a biological profile of the Neuroectoderm [9,10]. It was seen that Ameloblastomas showed strong positivity to CD99 and Synaptophysin, and equivocal positivity to S-100(100% soluble protein) and NSE. The study was based on the thought that the dental lamina originated from the Neuroectoderm.
To the best of our knowledge no prior studies have been done on normal oral ectoderm.
The positive reaction to all the neuroectodermal markers used in our study provides enough proof that the Ameloblastoma possesses an immunohistochemical profile similar to neuroectoderm and is consistent with the previous study [10].
The fact that the retromolar mucosa, the most likely representative zone also showed positive results as assumed, proves a logical connection between the neuroectoderm and the oral epithelium. It also makes it evident that the neuro ectodermal characteristic survives within the epithelium long after the initiating signals have seized.
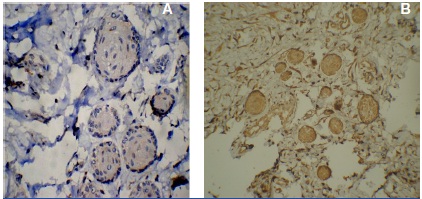
One of the retromolar tissue samples showed the presence of dental lamina rests which also were positive for all the markers providing an additional support to the study [Table/Fig-12,13, 14 a & b].
Ck19 staining in rests of serre 40x
Faint positivity to synaptophysin in rests of serre

CD99 and NSE positivity in the rests of serre
The positive staining results for the neuroectodermal markers in NRM and Ameloblastoma obtained in our study indicates that they both share a similar biological profile and therefore can be linked to each other. An interesting observation is that many peripheral Ameloblastomas show a direct continuity with the basal layer of the surface epithelium and this lends support to the hypothesis.
The fact that all the specimens used in study were positive for CK19 ensured a positive immune-reaction in the tissue, ruling out chances of fixation errors.
Conclusion
That there exists a potential for basal cells of the oral ectoderm, particularly that overlying the jaw bones to give rise to odontogenic cysts and neoplasms has had numerous supporters over the years and anecdotal occurrences like the proximity and apparent merger of peripheral Ameloblastomas to the basal cells of the overlying gingiva give credence to this theory. Establishing a link however has been difficult. The similarity in biologic characteristic between the two Embryologically unrelated tissues presented here seem to suggest that cells in the retromolar area tend to acquire and manifest neuroectodermal characteristics, a kind of ectodermal-mesenchymal transition, which carries on into the post odontogenic period of an individual. It also implies that this epithelium then possesses the capacity to proliferate and produce tumours of the odontogenic apparatus, particularly Ameloblastoma.
This has a clinical implication in the management of Ameloblastomas in the ascending ramus of the mandible, where mucosal stripping may have to be recommended as a mandatory rather than an elective procedure.
Additional studies with a wide sample range and more representative areas of oral tissues would lend a strong support to this study