Sertoli-Leydig Cell Tumour of Ovary with Menorrhagia: A Rare Case Report
Umesh Sidheshwar Kanade1, Sunita Sanjay Dantkale2, Rahul Ravindra Narkhede3, Rupali Ramrao Kurawar4, Shubhada Yadavrao Bansode5
1 Assistant Professor, Department of Pathology, Government Medical College and HospitalLatur, India.
2 Associate Professor, Department of Pathology, Government Medical College and HospitalLatur, India.
3 Assistant Professor, Department of Pathology, Government Medical College and HospitalLatur, India.
4 Junior Resident, Department of Pathology, Government Medical College and HospitalLatur, India.
5 Assistant Professor, Department of Pathology, Government Medical College and HospitalLatur, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Rupali Kurawar, Junior Resident (II), Department of Pathology, Government Medical College Latur-413512, India. Phone : 9561783827, E-mail :rupalikurawar@rediffmail.com
Sertoli-Leydig cell tumours (SLCTs) are rare sex cord stromal neoplasms of ovary accounting for less than 0.5% of all ovarian tumours. These are found in women of all age groups (2-75 y), but are most common in reproductive age group with an average age of 25 y. Mostly these are unilateral, confined to ovaries and usually stage I at the time of clinical diagnosis. The common presenting complaints in these patients are due to either mass occupying lesion (mostly pelviabdominal mass and/or pain) or hormonal production (mostly androgen and more rarely oestrogen). Androgenic manifestations, seen in 80% of patients with SLCT, are virilism, hirsutism, receding hairline, breast atrophy, clitoromegaly, acne, hoarseness of voice, etc. Estrogenic manifestations are precocious puberty, abnormal uterine bleeding, abnormal vaginal bleeding, menstrual irregularities, generalised oedema, weight gain, breast hypertrophy, endometrial hyperplasia, endometrial polyps and endometrial carcinoma. Histologically these are classified (WHO) as well-differentiated, intermediately differentiated, poorly differentiated, with heterologous components and retiform type. Prognosis depends upon degree of tumour differentiation (grading) and tumour extent (staging). We herein report an unusual case of SLCT of ovary with oestrogenic manifestation of menorrhagia.
Menorrhagia, Sertoli-leydig cell tumour, Sex cord stromal tumour
Case Report
A 28-year-old female, presented with pain in abdomen since 15 days and with a history of menorrhagia since two years. Per vaginal examination revealed a left adnexal mass. There were no clinical features suggesting virilisation. USG and CT scan of abdomen and pelvis revealed a left sided large ovarian neoplasm. On laparotomy a large left ovarian mass was noted. A specimen of total abdominal hysterectomy with unilateral salpingo-oopherectomy was received.
Grossly, a solid left ovarian mass of size 9x7x3.5 cm with smooth and lobulated external surface was noted. Cut section was solid, homogenous, yellow to tan in colour [Table/Fig-1]. Uterus and fallopian tube were unremarkable.
Solid ovarian mass with homogenous yellow to tan cut surface

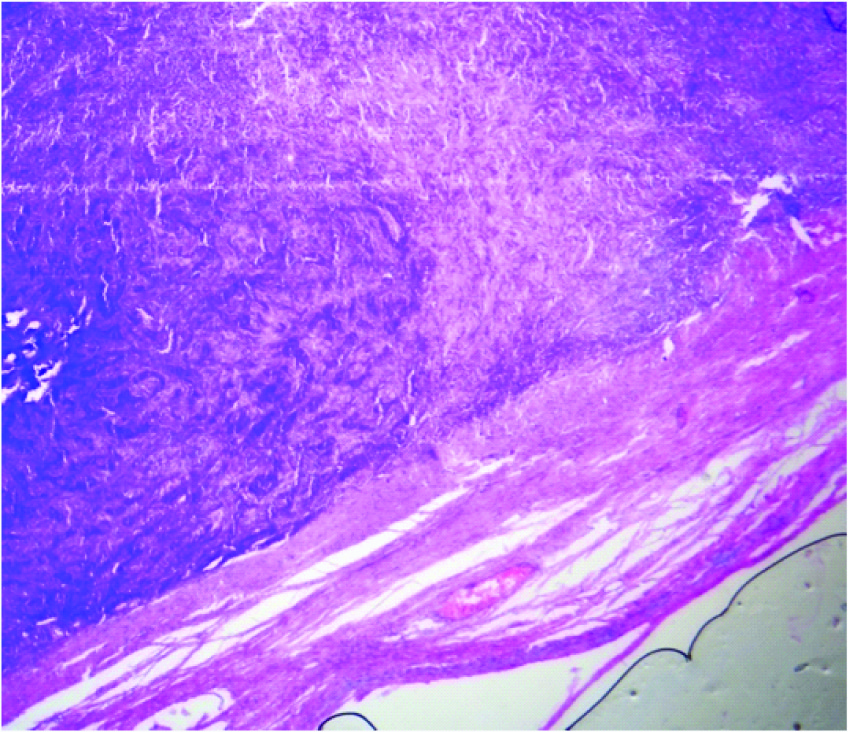
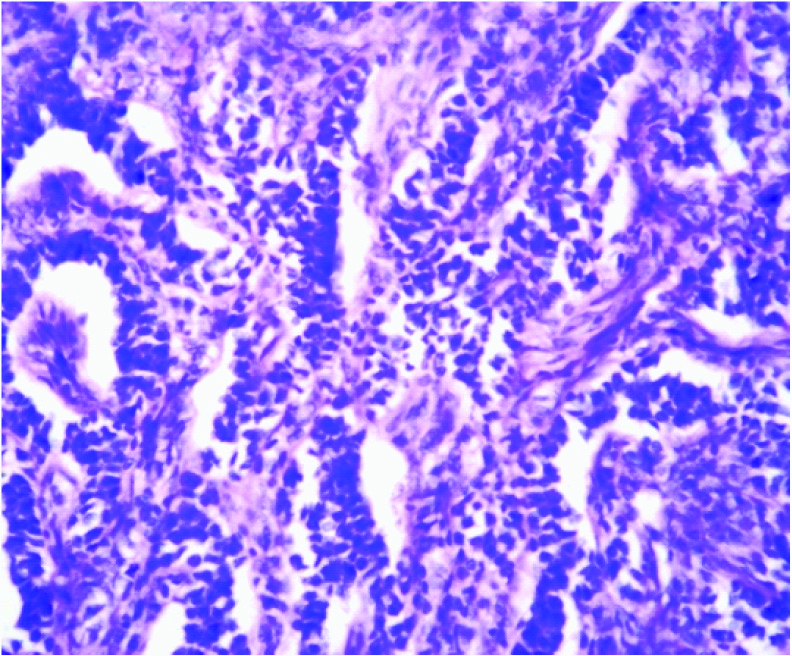
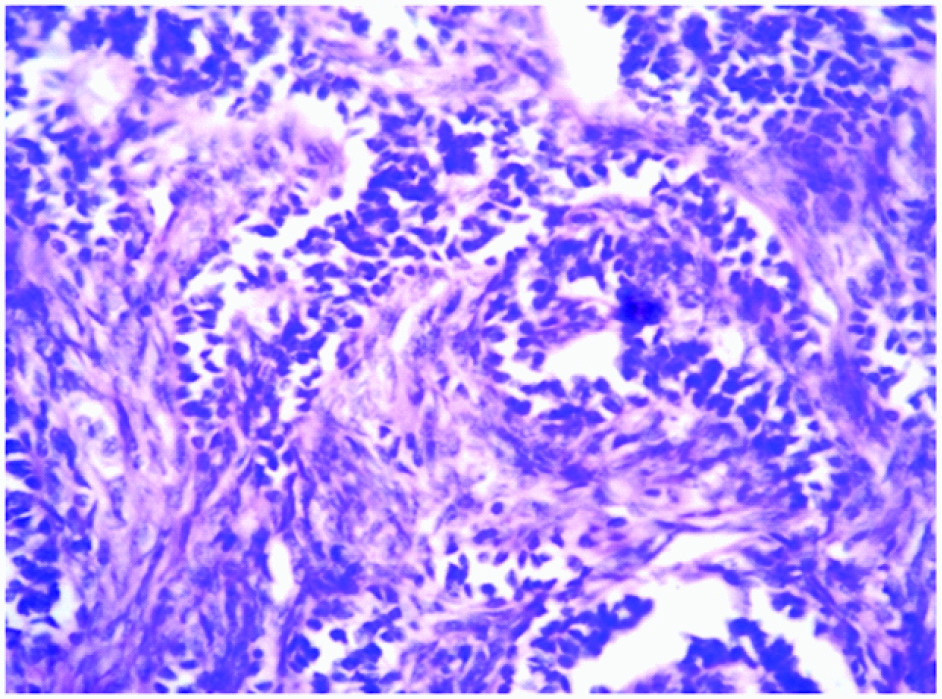
Microscopy revealed an encapsulated tumour [Table/Fig-2] composed of sertoli cells arranged in cords and trabeculae separated by abundant fibrous stroma [Table/Fig-3]. The individual tumour cells were oval with dark blue nucleus and eosinophilic cytoplasm. Adjacent to these, leydig cells were seen arranged in clusters and diffuse sheets, individual cells were having pale, eosinophilic to vacuolated cytoplasm. No mitotic figures were seen [Table/Fig-4]. On histopathology the diagnosis of Sertoli-Leydig cell tumour of left ovary with intermediate differentiation was given. The tumour was confined to one ovary with intact capsule hence stage IA was given according to the staging classification of International Federation of Gynaecology and Obstetrics.
Microscopic picture showing fibrous capsule with a tumour mass (H & E, 4x)
Microscopic picture showing cords and trabeculae of sertoli cells separated by abundant fibrous stroma (H & E, 10x)
Microscopic picture showing immature Sertoli cells having dark blue nucleus with adjacent Leydig cells having abundant pale eosinophilic to vacuolated cytoplasm (H & E, 40x)
Discussion
SLCT, a rare sex cord stromal tumour of ovary is suggested to be arising either from gonadal mesenchyme of ovary or from remnants of hilum [1]. Patients with SLCT present most commonly in second and third decades of life [2–5]. These are usually unilateral but bilateral tumours can be seen in < 2% of cases [2,4,6–8].
Clinical presentation can be due to either hormonal production or presence of mass occupying lesion [2]. SLCT can be inactive but elevated serum testosterone level is found approximately in 80% of patients [2]. More rarely oestrogen excess can be seen [2]. In our case a 28-year-old female presented with pain in abdomen and though virilising symptoms are common at presentation, sometimes exceedingly rare estrogenic manifestation such as menorrhagia can be seen as in our case. Our case correlated with study of Young and Scully, who studied 207 cases of SLCT with only one case presenting with menorrhagia [7,9].
Grossly these are typically firm, lobulated, yellow to tan solid masses with smooth external surface [7]. Cysts can be seen in tumours containing heterologous elements. Tumours with retiform component are soft, spongy, cystic with large, oedematous intraluminal polypoidal excrescences. Haemorrhage, necrosis and capsular rupture are common in poorly differentiated tumours [2,4,6]. The tumour size varies from 5-15 cm [4,6]. In our case, the tumour was unilateral, entirely solid, homogenous with intact capsule and yellow to tan on cut surface.
Histologically, SLCTs are classified (WHO) as 1) Well-differentiated (11%), 2)Intermediately differentiated (54%), 3)Poorly differentiated (13%), 4)With heterologous elements (22%) and 5) Retiform type (15%) [2,6,7,10]. Histopathologically well differentiated tumours are composed of tubules lined by sertoli-like cells separated by variable number of leydig cells. Intermediate type is characterized by cords, sheets and aggregates of sertoli-like cells separated by spindle stromal cells and recognizable leydig cells. Poorly differentiated tumours show masses of spindle shaped cells arranged in sarcomatoid pattern [11]. Tumours with heterologous elements are composed of either of the two types of components - a) endodermal elements comprising of gastric and intestinal mucin secreting epithelium and b) mesenchymal elements represented by immature cartilage or skeletal muscle [3,10]. In retiform category the SLCT co-exist with formations resembling rete of testis and ovary [11]. Immunohistochemically, SLCTs stain positive with inhibin, calretinin, WT1, CD56 and negative with epithelial membrane antigen (EMA) [2]. Histopathological grading of our case was SLCT with intermediate differentiation.
The main differential diagnoses are sertoliform variant of endometroid carcinoma, primary or metastatic carcinoid tumours and trabecular pattern of granulosa cell tumours (GCT). In endometroid carcinomas the neoplastic glands are small and tubular which may simulate the pattern of sex-cord stromal tumours particularly SLCTs. An old age patient with absence of endocrine manifestations, occurrence somewhere in the tumour of large tubular glands, squamous metaplasia, intraluminal mucous secretions suggest the diagnosis of endometroid carcinoma. Immunohistochemically, endometroid carcinoms are positive for EMA and keratin while they are negative for inhibin which is typically positive in SLCT. Carcinoid tumours can be differentiated by their round regular nuclei, a coarse chromatin pattern, moderately abundant cytoplasm and positivity for neuron-specific enolase, chromogranin and argentaffin or argyrophil reaction. The trabecular pattern of GCT can be differentiated from SLCT by the presence of more mature appearing cells than SLCT with angulated or deeply intended nuclei while SLCT shows radial orientation of nuclei in cords with Leydig cells in the adjacent stroma [1,11,12].
Prognosis of ovarian SLCT is significantly correlated with degree of tumour differentiation (grading) and tumour extent (staging) [2,4–8,13]. The malignant potential of well differentiated tumour is 0-1%, intermediately differentiated tumour is 11% and poorly differentiated tumour is 59% [2,4–6,8]. Metastasis is usually found in lungs, scalp, supraclavicular lymph nodes and liver [6,8]. In our case a 28 y female having left ovarian SLCT of intermediate differentiation and stage IA at presentation in keeping with reported series in which more than 90% of Sertoli-Leydig cell tumours are stage I at presentation [2,14].
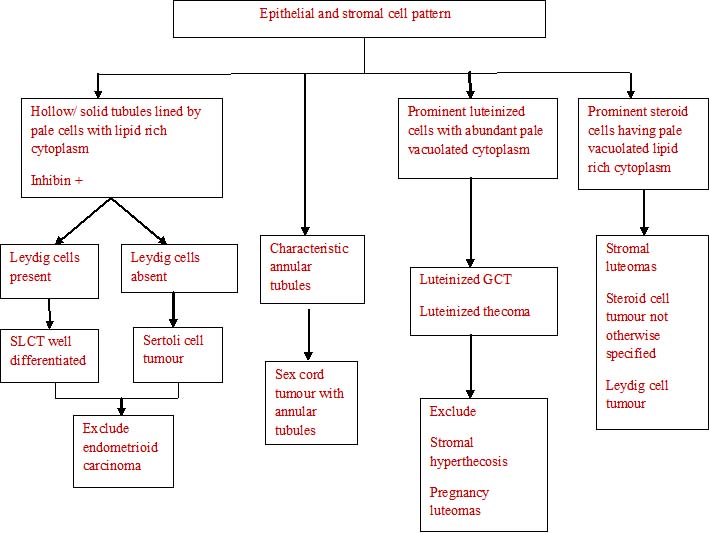
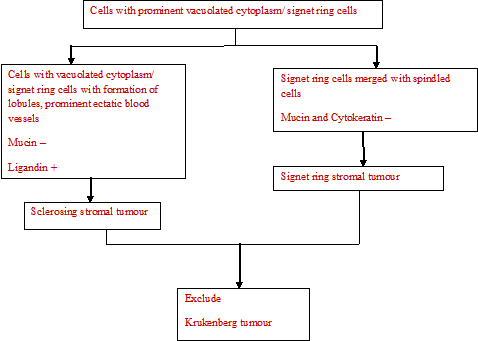
The histopathological algorithm depicting general approach to ovarian sex cord stromal tumours is given in flow charts [Table/Fig-5,6,7,8].
Pure mesenchymal/ stromal cell pattern
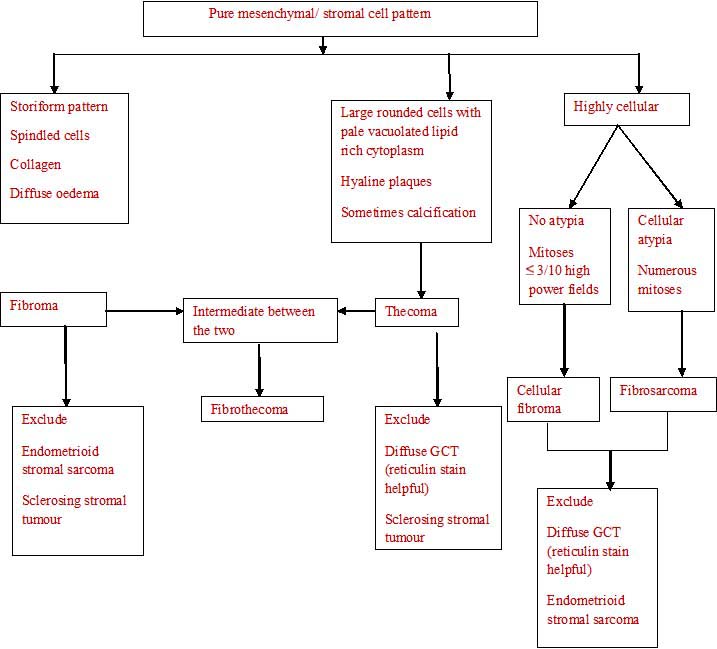
Pure epithelial/ epithelial and mesenchymal cell pattern
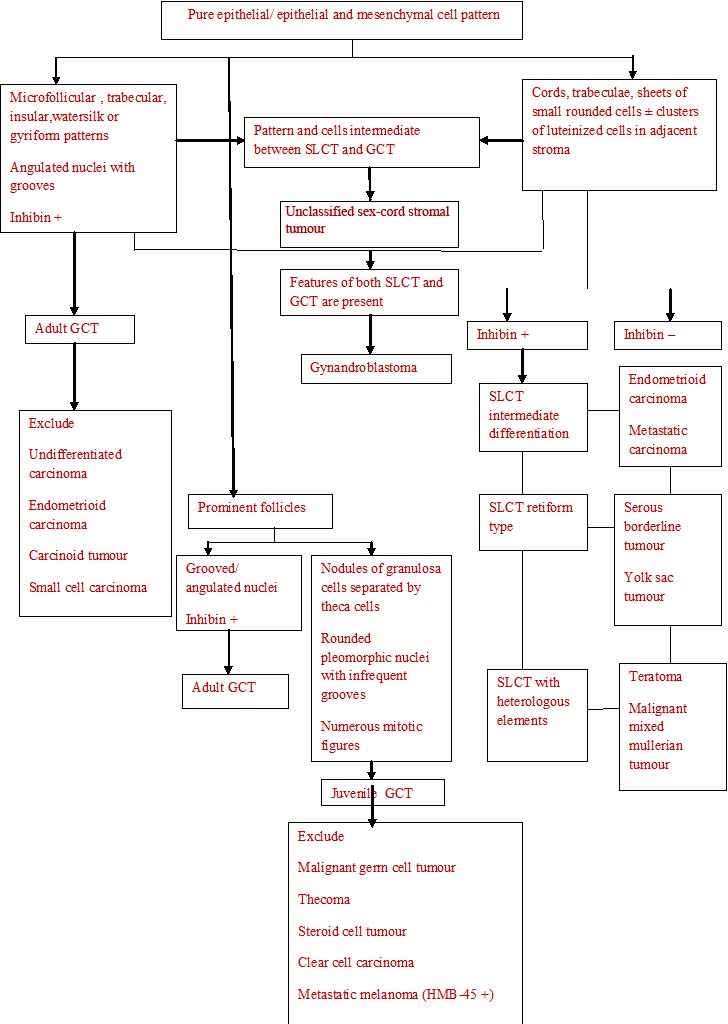
Epithelial and stromal cell pattern
Cells with prominent vacuolated cytoplasm/ signet ring cells
Conclusion
Though Sertoli-Leydig cell tumour of ovary most commonly present with androgenic manifestations, rarely it can also present with estrogenic manifestations as in our case. Therefore, each case of abdominal mass with estrogenic manifestations should be thoroughly investigated keeping Sertoli-Leydig cell tumour in mind which has good prognosis if detected at stage I.
[1]. Roth LM, Anderson MC, Govan ADT, Langley FA, Sertoli-Leydig Cell Tumors: A clinicopathologic study of 34 casesCancer 1981 48:187-97. [Google Scholar]
[2]. Abu-Zaid A, Azzam A, Alghuneim LA, Poorly differentiated ovarian Sertoli-Leydig cell tumor in a 16-year-old single woman: a case report and literature reviewCase Rep Obstet Gynecol 2013 2013:858501 [Google Scholar]
[3]. Tandon R, Goel P, Saha PK, Takkar N, Punia RP, A rare ovarian tumor - Sertoli- Leydig cell tumor with heterologous elementMedGenMed 2007 9(4):44-47. [Google Scholar]
[4]. Chand A, Pant R, Sertoli-Leydig cell tumor of ovary: A Case ReportMedical J of Shree Birendra Hospital 2011 10(2):35-37. [Google Scholar]
[5]. Siyambalapitiya S, Fernado J, Rajapakse RNG, Sertoli-Leydig Cell Tumor : a rare cause of androgenic alopecia in post-menopausal womenSri Lanka J of Diabetes, Endocrinology and Metabolism 2012 2(2):111-13. [Google Scholar]
[6]. Tayade S, Shivkumar PV, Malignant Sertoli Ledig Cell Tumor of Ovary in a Young AdolescentIJBR 2012 3(4):226-28. [Google Scholar]
[7]. Bhat RA, Lim YK, Chia YN, Yam KL, Sertoli leydig cell tumor of ovary: Analysis of a single institution databaseJ Obstet Gynaecol Res 2013 39(1):305-10. [Google Scholar]
[8]. Alam K, Maheshwari V, Rashid S, Bhargava S, Bilateral sertoli-leydig cell tumor of the ovary: A rare case reportIndian J of Pathol Microbiol 2009 52(1):97-99. [Google Scholar]
[9]. Young RH, Scully RE, Ovarian Sertoli-Leydig cell tumors: A clinicopathological analysis of 207 casesAm J Surg Pathol 1985 9(8):543-69. [Google Scholar]
[10]. Manjeera L, Vyas NM, Rai S, A case of ovarian Sertoli-Leydig cell tumor with multiple endocrine gland involvementInternational J of Reproduction, Conception, Obstetrics and Gynecology 2012 1(1):55-57. [Google Scholar]
[11]. Rosai J, Ackeaman. Surgical Pathology 2012 10th editionNew YorkElsevier Mosby [Google Scholar]
[12]. Sternberg Mills SE, Carter D, Diagnostic Surgical Pathology 2010 5th editionPhiladelphiaLippincott Williams and Wilkins [Google Scholar]
[13]. Desai VR, Dave KS, Mankad MH, Dave PS, Bhansali RP, Desai AD, Sertoli-Leydig cell tumors of ovaryThe J of Obstetrics and Gynecology of India 2009 59(2):165-67. [Google Scholar]
[14]. Ayhan A, Tuncer ZS, Hakverdi AU, Yuce K, Sertoli leydig cell tumor s of ovary: A clinicopathologic study of 10 casesEur J Gynaecol Oncol 1995 17:75-78. [Google Scholar]