Odontogenic lesions are a heterogeneous group of pathologies derived from epithelial and/or mesenchymal elements that are a part of the tooth-forming apparatus. They show varied behaviour ranging from benign cystic lesions (e.g. dentigerous cyst), benign locally aggressive lesions like ameloblastoma and keratocystic odontogenic tumour. Epithelial odontogenic tumours like ameloblastic carcinoma show malignant changes in the epithelial cells involving their proliferation, movement and growth into the connective tissue [1].
Type IV collagen along with laminin, plays an important role in cell adhesion, migration, differentiation, and growth. Its degradation products also play an important role during angiogenesis, tissue remodeling, and cancer progression [2]. Type IV collagen, being the major component of basement membrane (BM), demonstrates a stage- and position-specific distribution of its isoforms during tooth development [3].
Cell motility plays an important role in embryonic tissue remodeling, wound healing, angiogenesis, immune defense, invasion and metastasis. In normal physiological status, motility is tightly regulated, whereas tumour cell motility may be aberrantly regulated or auto regulated. Tumour-secreted or autocrine factors, like AMF or autotaxin, may be involved in this autoregulation [4]. Whether Collagen IV and Autocrine Motility Factor Receptor (AMFR) play an important role in the different odontogenic tumours is still unknown. Thus, the following study was carried out to determine and evaluate the expression of type IV Collagen and AMFR in odontogenic tumours.
Materials and Methods
Thirty one cases of previously diagnosed odontogenic tumours including KCOT, multicystic Ameloblastoma, unicystic ameloblastoma and ameloblastic carcinoma based on clinical, radiographic and histopathological findings were retrieved from the archives of the Department of Oral Pathology, MM College of Dental Sciences and Research, Mullana, India. Tissues with intense inflammation or tissue sections which did not have sufficient tumour tissue were excluded from the study. Two – 3μ sections were prepared on Poly-L-Lysine coated slides and stained immunohistochemically with type IV collagen antibody and AMFR antibody in all cases. Immunohistochemical staining for type IV collagen was performed using mouse type IV Anti - collagen monoclonal antibody; isotype IgG1, (Biogenix, India) overnight at 40C followed by secondary antibody for 30 min. 2.5 mg/ml pepsin digested enzyme (Biogenex chemical, Milmont Drive, Fremont, USA, EK000-5KE) was used for optimal antigen retrieval for 7 min at 370C. Negative controls with an omission of the antiserum from the primary incubation were also included. Stained blood vessels in the connective tissue were taken as an internal positive control. Immunohistochemical staining for AMFR was performed using autocrine motility factor receptor monoclonal antibody (Life Technology, India) for 30 min incubated in a moist petri dish followed by secondary antibody for 30 min. Antigen retrieval was performed in EZ Retriever system. Negative controls with an omission of the antiserum from the primary incubation were included. Oral squamous cell carcinoma was taken as a positive control. Imaging analysis system (Nikon Research Microscope, ECLIPSE 80i) was used to assess the staining patterns. Three observers recorded the findings of the same slides to eliminate the inter observer differences. The observers evaluated the slides at intervals to remove the intra observer bias.
Criteria for scoring
Type IV Collagen: The evaluation of positive reaction was performed using the criteria modified from Zuk RJ et al., [5]. Fenyvesi A [6] and Arduino PG et al., [7]. Basement membrane staining was evaluated semi quantitatively and graded as Absent staining (score zero); Minimal staining, when present in (1% to 20%) of basement membrane (score one); large interruption, when (21% to 50%) of basement membrane staining was evident (score two); Focal interruption, when greater than (50%) of basement membrane staining was evident (score three); Completeness of staining, discrete staining (score four); Completeness of staining, intense staining (score five).
AMFR was evaluated as a product of the qualitative and quantitative scores based on the criteria given by Rodrigo JP et al., [8]. The qualitative criteria were determined by positively stained cells based on intensity of staining as no staining (score zero); mild staining (score one); moderate staining (score two) and intense staining (score three). The quantitative criteria were determined by positively stained cells as no staining (score zero), 1%-10% of tumour cells positive (score one), 11%-50% of tumour cells positive (score two) and >50% tumour cells positive (score three). The final score (product of qualitative and quantitative scores) was given as 0 if the product was 0; mild for scores 1-3; moderate for scores 4-7 and intense for scores 8 or more. Data was further subjected to statistical analysis. Means and standard deviations for type IV collagen and AMFR score were calculated for all the groups [Table/Fig-1]. Statistical analysis was performed using One-way ANNOVA and significant difference was assessed by LSD Correlation test using SPSS 13.0 software.
Results of Type IV Collagen staining and AMFR in the various odontogenic lesion
| N | TYPE IV Collagen | AMFR |
|---|
| Mean | Std. Deviation | Std. Error | Mean | Std. Deviation | Std. Error |
|---|
| Unicystic Ameloblastoma | 5 | 1.4000 | 1.34164 | .60000 | 4.0000 | 1.87083 | .83666 |
| Multicystic Ameloblastoma | 6 | 2.3333 | .81650 | .33333 | 1.3333 | 1.03280 | .42164 |
| Keratocystic Odontogenic Tumour | 9 | .5556 | .52705 | .17568 | 5.0000 | 1.93649 | .64550 |
| Ameloblastic Carcinoma | 3 | 1.6667 | 1.52753 | .88192 | 9.0000 | .00000 | .00000 |
| Normal Follicular Tissue | 8 | 1.7500 | 1.16496 | .41188 | 3.1250 | 2.16712 | .76619 |
| Total | 31 | 1.4516 | 1.15004 | .20655 | 4.0323 | 2.67686 | .48078 |
Results
Maximum mean expression for Type IV Collagen was shown by multicystic ameloblastoma (2.333±0.816); and minimum by keratocystic odontogenic tumour (0.5556±0.527) [Table/Fig-1]. In case of expression for AMFR maximum mean expression was shown by ameloblastic carcinoma (9.000±0.00); and minimum expression by multicystic ameloblastoma (1.333±1.032) [Table/Fig-1]. For type IV Collagen significant difference was observed only between multicystic ameloblastoma and keratocystic odontogenic tumour (p-value 0.003) and between keratocystic odontogenic tumour and normal follicular tissue (p-value 0.023) [Table/Fig-2].
Correlation between different groups of odontogenic lesions for type IV Collagen staining. *-significant statistical difference
| (I) lesion | (J) lesion | Mean Difference (I-J) | Std. Error | Sig. |
|---|
| Unicystic Ameloblastoma | Multicystic Ameloblastoma | -.93333 | .61618 | .142 |
| KCOT | .84444 | .56758 | .149 |
| Ameloblastic Carcinoma | -.26667 | .74314 | .723 |
| Normal | -.35000 | .58011 | .552 |
| Multicystic Ameloblastoma | Unicystic Ameloblastoma | .93333 | .61618 | .142 |
| KCOT | 1.77778(*) | .53631 | .003 |
| Ameloblastic Carcinoma | .66667 | .71954 | .363 |
| Normal | .58333 | .54956 | .298 |
| Keratocystic Odontogenic Tumour (KCOT) | Unicystic Ameloblastoma | -.84444 | .56758 | .149 |
| Multicystic Ameloblastoma | -1.77778(*) | .53631 | .003 |
| Ameloblastic Carcinoma | -1.11111 | .67839 | .113 |
| Normal | -1.19444(*) | .49446 | .023 |
| Ameloblastic Carcinoma | Unicystic Ameloblastoma | .26667 | .74314 | .723 |
| Multicystic Ameloblastoma | -.66667 | .71954 | .363 |
| KCOT | 1.11111 | .67839 | .113 |
| Normal | -.08333 | .68891 | .905 |
| Normal Follicular Tissue | Unicystic Ameloblastoma | .35000 | .58011 | .552 |
| Multicystic Ameloblastoma | -.58333 | .54956 | .298 |
| KCOT | 1.19444(*) | .49446 | .023 |
| Ameloblastic Carcinoma | .08333 | .68891 | .905 |
For AMFR significant difference was observed between unicystic ameloblastoma and multicystic ameloblastoma (p-value 0.20); unicystic ameloblastoma and ameloblastic carcinoma (p-value 0.001); multicystic ameloblastoma and keratocystic odontogenic tumour (p-value 0.001); multicystic ameloblastoma and ameloblastic carcinoma (p-value 0.000); KCOT and Ameloblastic carcinoma (p-value 0.002); keratocystic odontogenic tumour and normal follicular tissue (p-value 0.039); and between ameloblastic carcinoma and normal follicular tissue (p-value 0.000) [Table/Fig-3].
Correlation between different groups of odontogenic lesions for AMFR staining. *-significant statistical difference
| (I) lesion | (J) lesion | Mean Difference (I-J) | Std. Error | Sig. |
|---|
| Unicystic Ameloblastoma | Multicystic Ameloblastoma | 2.66667(*) | 1.07673 | .020 |
| KCOT | -1.00000 | .99181 | .323 |
| Ameloblastic Carcinoma | -5.00000(*) | 1.29859 | .001 |
| Normal | .87500 | 1.01371 | .396 |
| Multicystic Ameloblastoma | Unicystic Ameloblastoma | -2.66667(*) | 1.07673 | .020 |
| KCOT | -3.66667(*) | .93717 | .001 |
| Ameloblastic Carcinoma | -7.66667(*) | 1.25735 | .000 |
| Normal | -1.79167 | .96032 | .073 |
| Keratocystic Odontogenic Tumour (KCOT) | Unicystic Ameloblastoma | 1.00000 | .99181 | .323 |
| Multicystic Ameloblastoma | 3.66667(*) | .93717 | .001 |
| Ameloblastic Carcinoma | -4.00000(*) | 1.18544 | .002 |
| Normal | 1.87500(*) | .86403 | .039 |
| Ameloblastic Carcinoma | Unicystic Ameloblastoma | 5.00000(*) | 1.29859 | .001 |
| Multicystic Ameloblastoma | 7.66667(*) | 1.25735 | .000 |
| KCOT | 4.00000(*) | 1.18544 | .002 |
| Normal | 5.87500(*) | 1.20382 | .000 |
| Normal Follicular Tissue | Unicystic Ameloblastoma | -.87500 | 1.01371 | .396 |
| Multicystic Ameloblastoma | 1.79167 | .96032 | .073 |
| KCOT | -1.87500(*) | .86403 | .039 |
| Ameloblastic Carcinoma | -5.87500(*) | 1.20382 | .000 |
Discussion
Type IV Collagen forms an important component of the basement membrane in the early stages of the development of the dental organ and has been known to be secreted by the pre-ameloblasts. With the deposition of the first layer of predentin; type IV collagen is lost in the basement membrane between enamel organ and dental papilla with an increase in expression in Type I Collagen (secreted by connective tissue fibroblasts) in this area.
In the present study, type IV Collagen staining was observed in the basement membrane region ranging from incomplete staining with large or focal interruptions to staining being absent in some lesions; a complete staining without any focal or large interruptions was not observed in any of the cases [Table/Fig-4]. In contrast to our study J Hangelbroek et al., [9] failed to observe the presence of type IV Collagen in any of the nineteen samples of odontogenic tumours.
Expression of Type IV collagen in odontogenic lesions. (A) unicystic ameloblastoma - mild staining, large interruptions. (B) Multicystic ameloblastoma - intense staining; Focal interruptions. (C) keratocystic odontogenic tumor - minimal staining, basement membrane (D) ameloblastic carcinoma - patchy areas - intense staining; large interruptions
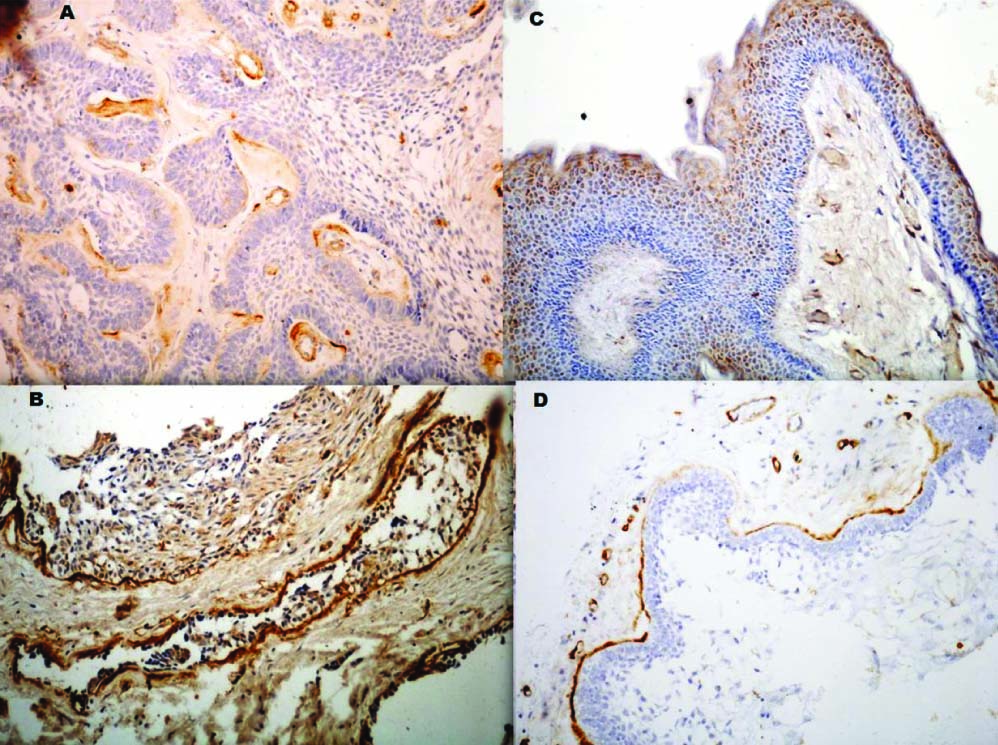
The presence of collagen IV in basement membrane of multicystic ameloblastoma [Table/Fig-4a] may be attributed to the presence of ameloblast like cells which resemble the cells of inner enamel epithelium that have the inherent ability to secrete collagen IV. Nakano K et al., [10] in their study suggested that alpha-4 (Collagen IV) expression was rare in odontogenic neoplasms and occurred around nests of tumour cells and potentially invasive sites suggesting an important role in cytodifferentiation and progression. In the present study the tumour islands at the advancing front of the tumour showed minimal collagen IV staining in the basement membrane, suggesting the association of loss of type IV Collagen expression with progression of lesion. Also, Zhang B et al., [11] showed that the degradation of type IV Collagen by MMP-2 and MMP-14 may contribute to local invasive characteristics of ameloblastoma. But in a study done by Sauk JJ [12], all the tumour islands of ameloblastomas were circumferentially delineated by a linear staining to both anti-basement membrane antibodies (Type IV Collagen and laminin), hence suggesting that ameloblastoma spread into tissue spaces by expanding their compartmental volumes rather than by secreting proteinases that disrupt the basement membranes compartmental barriers. In the present study, desmoplastic ameloblastoma showed a higher expression of Collagen IV as compared to the other variants [Table/Fig-4b]. Phuu Pwint Han et al., [13] also found a higher expression of type IV collagen in desmoplastic ameloblastoma in comparison to the other variants of ameloblastoma. In the present study two out of five cases of unicystic ameloblastoma showed a total absence of collagen IV expression. These two cases showed the invasion of ameloblastoma cells in the form of interlacing strands into the underlying connective tissue. One case of unicystic ameloblastoma showed positive expression type IV collagen staining in the basement membrane region with only focal interruptions. In this case, the tumour cells did not show any infiltration into the underlying connective tissue thus being a luminal variant of ameloblastoma.
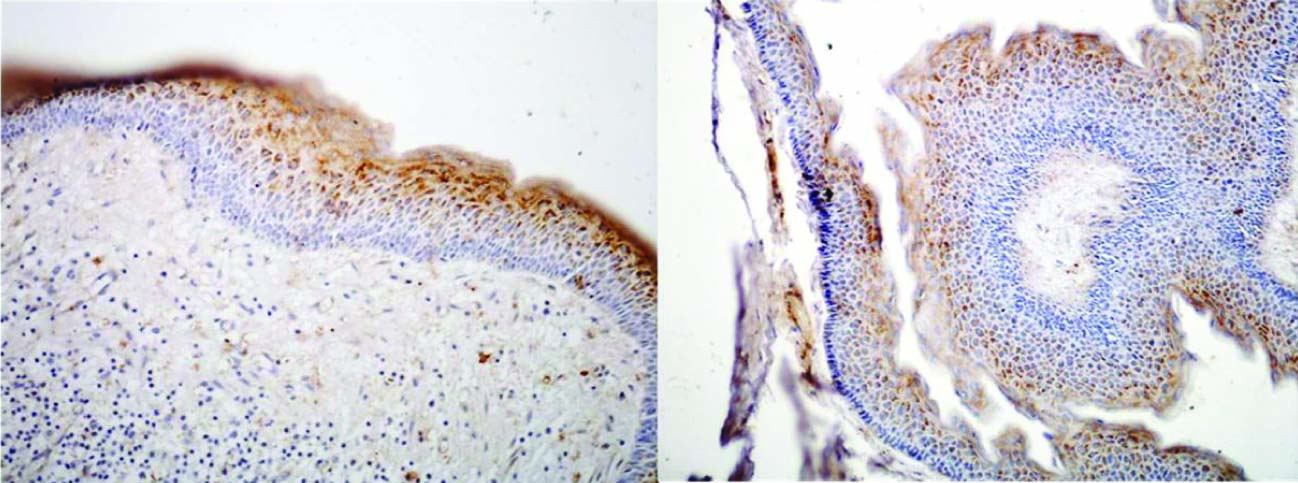
A significantly lower expression of type IV collagen was seen in the basement membrane zone in KCOT [Table/Fig-4c] compared to Multicystic ameloblastoma. Philipsen et al., [14] demonstrated that juxta-epithelially, deep to the lamina densa, the collagen showed signs of dissolution and often disappeared completely. They suggested that this process of collagenolysis might be produced by a collagenase or other proteases and it appears to be this phenomenon that is responsible for the ready separation of OKC epithelium from its supporting capsule. Amorim RFB et al., [15] correlated the negative expression of type IV Collagen in basement membrane of Nevoid Basal Cell Carcinoma Syndrome (NBCCS) related KCOT with the aggressive biological behaviour of KCOT. Amaral FR et al., [16] studied the cell proliferative index and apoptotic index in KCOT and found that both the indices of KCOT were significantly higher in cells of the basal and supra-basal layers, hence supporting its aggressive behaviour. In the present study positive granular cytoplasmic staining was seen within epithelial cells of KCOT lining with increase in intensity towards the superficial epithelial layers and in the corrugated parakeratinized layer [Table/Fig-5]. This may suggest a specific nature of the corrugated parakeratin and highlight its difference from the parakeratin laid down by the oral squamous epithelial cells.
Expression of Type IV Collagen in Keratocystic odontogenic tumor. Positive granular cytoplasmic staining is seen within epithelial cells with increase in intensity towards the superficial epithelial layers and in the corrugated parakeratinized layer
All the cases of ameloblastic carcinoma showed patchy areas of intense staining of type IV collagen with large interruptions. The areas without collagen IV staining synchronized with areas of highly proliferating malignant cells [Table/Fig-4d]. Nagatsuka H et al., [3] performed a study to determine localization of collagen IV in basement membrane of odontogenic neoplasms using six anti-α (IV) chain-specific monoclonal antibodies and suggested that modification and remodeling of basement membrane collagen IV α chains occur during progression of odontogenic neoplasms. Tanjore H et al., [2] stated that normal assembly and organization of the basement membrane is disrupted in cancer progression, and suggested that such alterations in selective type IV Collagen expression are likely regulated at the gene level by hypemethylation events in malignancy.
In the present study, a variable expression of AMFR was observed in the odontogenic lesions ranging from intense expression to negative expression [Table/Fig-6]. Ameloblastic carcinoma showed a very high expression of AMFR. Unicystic ameloblastoma and Keratocystic odontogenic tumour also showed a significant expression. Multicystic ameloblastoma showed a negative to mild expression only.
Expression of AMFR in odontogenic lesions. (A) multicystic ameloblastoma (B) unicystic ameloblastoma (C) keratocystic odontogenic tumor with faint cytoplasmic expression, increasing towards the basal layers. (D) ameloblastic carcinoma - intense expression; distinct intense nuclear expression - peripheral ameloblasts
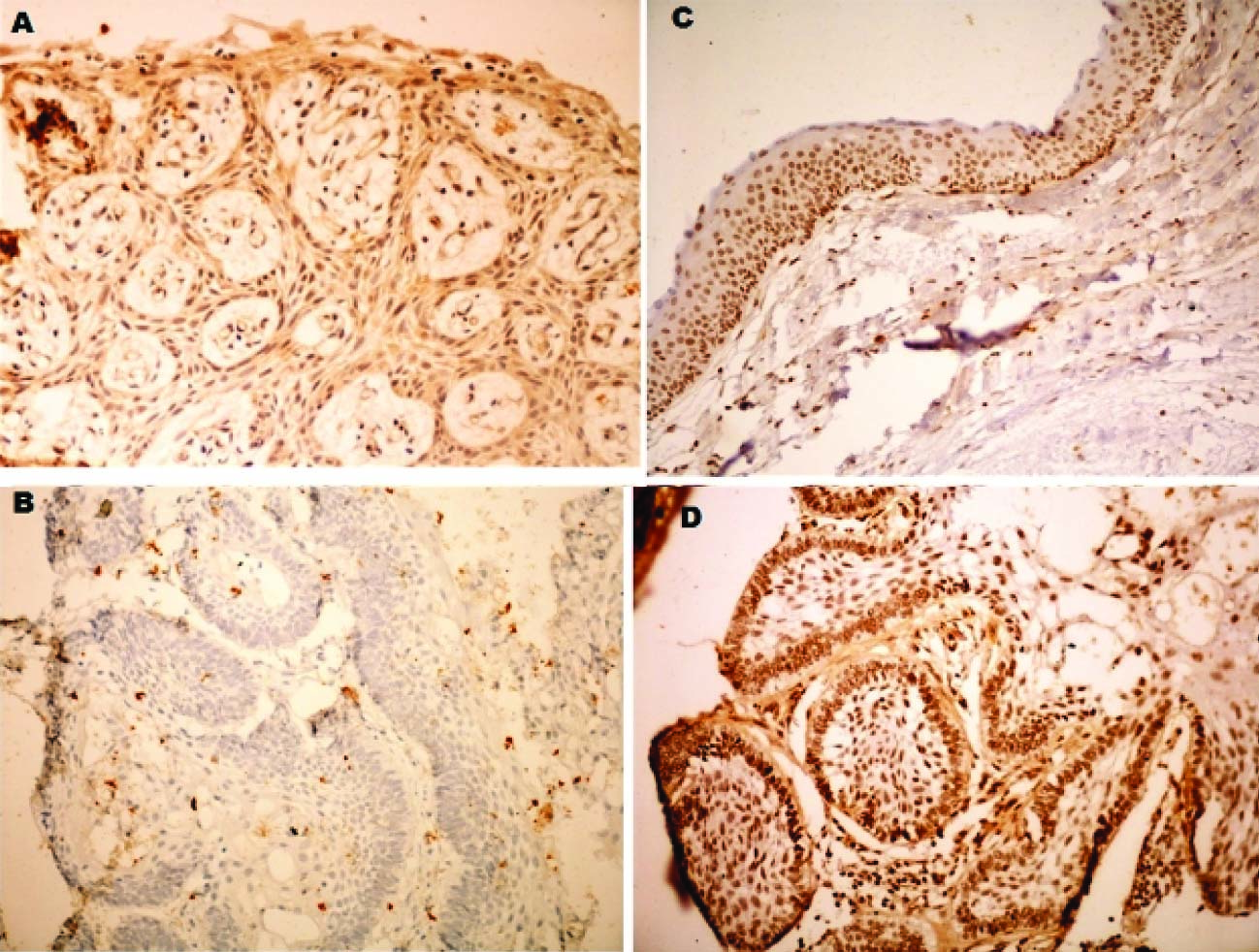
Normal follicular tissue showed no or minimal activity with AMFR. Nakamori S et al., [17] and Maruyama et al., [18] also reported absence of significant expression of AMFR in normal tissues Thus, generally AMFR is shown to be minimally expressed in normal tissues.
The weak expression of AMFR in multicystic ameloblastoma [Table/Fig-6a] may be explained by the fact that, though locally invasive, multicystic ameloblastoma consists of tumour cells that resemble mature odontogenic epithelial cells, especially ameloblasts and stellate reticulum, which do not exhibit the property of motility. Also, the progression of this lesion is mainly by increase in the number of follicles and minimal invasion of the connective tissue.
In all cases of unicystic ameloblastoma, more than 50% of epithelial cells showed positive AMFR expression [Table/Fig-6b]. AMFR expression is raised in neoplastic epithelial cells which show proliferation into the connective tissue as motility may play an important role in the growth of such lesion and their transformation from benign quiescent lesions to aggressive ones. This may explain the consistent positive expression of AMFR in all cases of unicystic ameloblastoma as these tumours have an inherent tendency to transform into aggressive multicystic ameloblastoma by extension of neoplastic odontogenic epithelial cells into the connective tissue. The varying grades of positive AMFR expression in all cases of unicystic ameloblastoma may depend upon the nature of the lesion and the level of development of the tumour cells towards mature ameloblasts, since mature cells become tissue specific and exhibit less tendency to be motile whereas immature cells still show the property of motility. This study included one case of mural unicystic ameloblastoma which showed both nuclear and cytoplasmic expression [Table/Fig-7].
Nuclear and cytoplasmic AMFR expression in mural variety of unicystic ameloblastoma

KCOT showed a higher expression than both multicystic and unicystic ameloblastoma [Table/Fig-6c]. KCOT has been observed in previous studies to the more aggressive tumour with higher proliferative index, also the maturation level of ameloblastoma cells is higher than cells of KCOT. Except for two cases of KCOT which showed mild expression the rest eight cases showed moderate to intense expression. The upper epithelial layers stained intensely positive and the basal layers comprising of tall columnar cells showed a reduced expression. This can be owed to the higher differentiation of basal layers which resemble odontogenic epithelium. Consistent with this explanation is the reduced expression of peripheral cells of ameloblastoma with AMFR. Hence we can say that AMFR may not be expressed by epithelial cells showing some amount of differentiation. Hirono Y [4] observed that patients with undifferentiated type carcinomas had higher positive rates of AMFR expression than patients with differentiated type tumours.
Ameloblastic carcinoma showed a significantly higher expression of AMFR in the neoplastic epithelial cells when compared to all other lesions in this study. Also, a consistent intense nuclear expression was observed in this group [Table/Fig-6d]. Ameloblastic carcinoma comprises of malignant cells with the potential to metastasize showing the inherent characteristics of motility. All three cases showed intense expression with more than 90% of tumour cells showing positive expression. Niinaka Y et al., [19], Maruyama et al., [18] and Jiang WG [20] also observed that an increased expression of AMF/AMFR was associated with invasion and progression of malignant neoplasms.
Conclusion
A significant positive expression of Type IV Collagen was observed in Unicystic Ameloblastoma, Multicystic Ameloblastoma and Ameloblastic Carcinoma, whereas a negative expression was observed with Keratocystic Odontogenic Tumour in the basement membrane region, though, an intense expression with the superficial epithelial layers and corrugated parakeratin layer was observed. AMFR expression was found to be associated with the aggressive potential of tumours. Cells exhibiting certain levels of differentiation showed a decrease in expression inspite of having an aggressive nature as we observed in Multicystic ameloblastoma. The intense expression of ameloblastic carcinoma showed a correlation of AMFR expression with the invasive malignant tendency of the lesion.
Thus, the present study sheds new light on the potential aggressiveness of odontogenic lesions. These findings can significantly affect the clinical course and treatment of these lesions. Many studies have been performed on the proliferative potential of the odontogenic epithelial cells but this study emphasizes on its true aggressive and invasive nature too.
One of the major shortcomings of this study would be its limited sample size. A larger sample encompassing other odontogenic lesions would give us a broader perspective. Hence, this study is merely the first step in initiating a mile long run.