Endodontics has undergone a revolutionary change. Right from the stage of endodontic diagnosis till post endodontic restoration, every stage of treatment has witnessed the impact of technological advances. The precision with which the treatment can be carried out has made the prognosis quite predictable [1].
It has been established beyond doubt that bacteria play a decisive role in the development of endodontic lesions. Consequently, one of the major goals of a successful endodontic is the elimination of the bacteria from the root canal space and the removal of the substrate they depend on [2]. For years a plethora of substances have been advocated for use in root canal therapy. The historical origins of medicaments date back to very early times. Scribonius in 1045 AD wrote of using oils and wine in the mouth of patients in pain. Through the middle ages there are references of use of clove oil,beech wood creosote as medicaments [2].
Unfortunately, most of these medicaments were non-specific and by themselves caused discomfort to the patients.
In such a situation Calcium hydroxide has emerged as a potent intracanal medicament [1]. Ever since it’s introduction by Hermann in the beginning of the 20th century, it has gripped endodontics in a wide array of uses,
It’s various biological properties include, antibacterial activity, tissue dissolving ability and induction of repair by hard tissue formation. The main benefit lies in the antibacterial effect conferred by it’s high pH (12.5) which is a deterrent for the survival of micro-organisms [2].
Extra radicular microbial biofilms are the biofilms formed on the root(cementum) surface adjacent to the root apex of endodontically, infected teeth. They have been associated with periapical inflammation and delayed periapical healing inspite of adequate orthograde root canal treatment and ultimately lead to failure of endodontically treated teeth. In such clinical situations, calcium hydroxide when mixed with different vehicles has shown the potential to percolate calcium and hydroxyl ions through the cementum thus preventing colonization of bacteria in the apical and periradicular area [3,4].
The vehicle plays an important role in the overall process. It determines the velocity of ionic dissociation causing the paste to be solubilized and resorbed at various rates from the root canal as well as the periapical tissues. It has a direct relationship with the concentration and antibacterial activity. Thus an ideal vehicle should allow a gradual and slow release of calcium and hydroxyl ions.
The incorporation of calcium hydroxide (51-52%) in guttapercha points introduced by Coltene whaledent Inc have claimed to overcome the poor handling and incomplete removal of calcium hydroxide suspensions difficulties prior to obturation [6,7]. Hence this study was conducted to evaluate the pH variation in the surrounding medium after the use of various Ca(OH)2 preparations over a period of 7 d.
Materials and Methods
Sixty intact single rooted human premolars freshly extracted for orthodontic reasons from young adults were obtained for the present study. The teeth were free of caries, restorations, cervical abrasions and fractures. Any soft tissue, calculus covering the root surface was gently removed with scalers and the teeth were stored in distilled water until use.
Preparation of samples for evaluation
The teeth were decoronated at the cemento-enamel junction with carborundum discs in a high speed hand piece under a water spray. So that, the root length was maintained between 11 to 14 mm, for each specimen radiographs were taken mesiodistally and bucco-lingually to confirm the presence of a single canal. Extremely oval, kidney and ribbon shaped canals were excluded from the study. The working lengths of the root canals were determined by inserting a no.10 K- file uptill it was seen at the apex and then subtracting 1mm from it’s length. The canals were then enlarged by a conventional technique by instrumentation upto size 40 master apical file, with a constant irrigation of 1ml of 3% sodium hypochlorite followed by 1ml of normal saline.
External defects measuring 3mm in diameter and 1mm in depth were then prepared on the mesial surface using an end cutting bur at high speed in the coronal third of the root surface, Glyde was then dispensed in the root canals, followed by rinsing with 10ml of distilled water to remove the glyde completely. The root canals of all the teeth were then dried with sterile paper points before placement of any medications[Table/Fig-1,2,3,4].
Materials used for the present study
| 1. | Calcium hydroxide powder | Vensons dental products |
| 2. | Calcium hydroxide points | Hygienic, Coltene whaledent Inc |
| 3. | 10% propylene glycol | Nise chemicals |
| 4. | Saline solution | Baxter Pvt Ltd |
| 5. | 3% sodium hypochlorite | Vishal Dentocare |
| 6. | Cavit G | 3 M ESPE |
| 7. | Glyde File Prep | Dentsply / Maillefer |
| 8. | Nail varnish | |
| 9. | Distilled water | |
Composition of the material
| 1 | Calcium hydroxide points | Calcium hydroxide – 52% |
| Guttapercha – 42% |
| Sodium chloride |
| Surfactant |
| Colouring agents |
| 2 | 10% propylene glycol | Propane – 12, DIOL |
| CH3CH (OH) CH2OH = 76.10% |
| Water – 0.2% |
| 3 | Saline solution | Sodium chloride |
| Injection IP 0.9% w/v |
Picture of radiograph from mesiodistal and buccolingual direction to show the presence of single canal
Sample being decoronated at the cemento-enamel junction and maintained at 11-14mm
Groups 60 teeth were randomly assigned to four major groups. One control of 15 and the remaining three experimental groups of 15 each.
In Group I: The root canals were left empty and served as a control.
In Group II: The root canals were filled with a manually prepared paste of Ca(OH)2 powder and saline, 130 mg of Ca(OH)2 was mixed with 0.25 ml of saline to make a paste. The powder to liquid ratio was determined so as to incorporate maximum amount of Ca(OH)2 yet result in a thick creamy consistency paste. A no 20 K- file was used to apply the paste followed by the use of hand pluggers to compact the material. Radiographs were taken to confirm the homogencity of the paste fill and absence of voids in the root canals.
In Group III: The root canals were filled with a manually prepared paste of pure calcium hydroxide powder and propylene glycol. 130 mg of calcium hydroxide powder was mixed with 0.25ml of propylene glycol to make a paste. The powder to liquid ratio was determined so as to incorporate maximum amount of Ca(OH)2 yet result in a thick creamy consistency. A no 20 K-file was used to apply the prepared paste followed by use of hand pluggers to compact the material. Radiographs were taken to confirm the homogencity of the paste fill and absence of voids in the root canal.
In Group IV: The root canals were filled with single Ca(OH)2 point. The Ca(OH)2 points (#40) were placed according to the manufacturer’s instructions, i.e., corresponding to the last used instrument, passively in the canal, without applying any condensation uptil the working length.
The coronal access was filled with 3 mm of temporary filling material Cavit G. All the surfaces of root except the defects were covered with three coats of Nail varnish.
Each sample was then placed in airtight plastic vials containing 2ml of distilled water pH (7). The volume of the distilled water was chosen to suffice the requirements for the submergence of the electrode of the pH meter. The samples were kept at 37°C throughout the observation period in an incubator (Orbital Incubator compressed model No. 397IAG) [Table/Fig-5].
Final prepared experimental sample. External defect prepared on the mesial surface in the coronal third of the root (3mm in diameter and 1mm in depth)
pH measurements
The pH meter was calibrated with standard pH solutions of pH 4,6, and 9. The test tip was washed with distilled water and dried with tissue paper to prevent any contamination between the tests. An average of 3 reading was taken for one pH value. Precision of the apparatus was verified with constant measurements with the standard buffer. The pH of each sample was measured after 1,3,5 and 7 days respectively. The pH was measured using a digital pH meter, Model No 335,ELICO PRIVATE LTD HYDERABED INDIA [Table/Fig-6].
ELICO pH Meter Model No.335 with COMBINATION GLASS ELECTRODE in experimental vial

Results
Statistical analysis used
Results were statistically analyzed using repeated measure ANOVA, independent ‘t-tests (independent and paired sampling) and Scheffe’s post hoc test.
All the statistical methods were carried out using SPSS(16.0) windows software.
A statistically significant difference (p<0.05) existed between the experimental groups over the observation period with day 5 recording the highest pH of 9.7259 and least at day 1 (8.9364).
At day 1, a high pH was recorded by the Calcium hydroxide points and Saline groups and a lower pH by the Propylene glycol group. At day’s 3 and 5, the maximum pH was recorded by the Propylene glycol group and minimum by the Calcium hydroxide points and Saline groups. At day 7, the pH of all the groups had dropped, with Propylene glycol recording the maximum pH followed by saline and lastly Calcium hydroxide points [Table/Fig-7,8].
Mean pH values of all the groups over the observation period
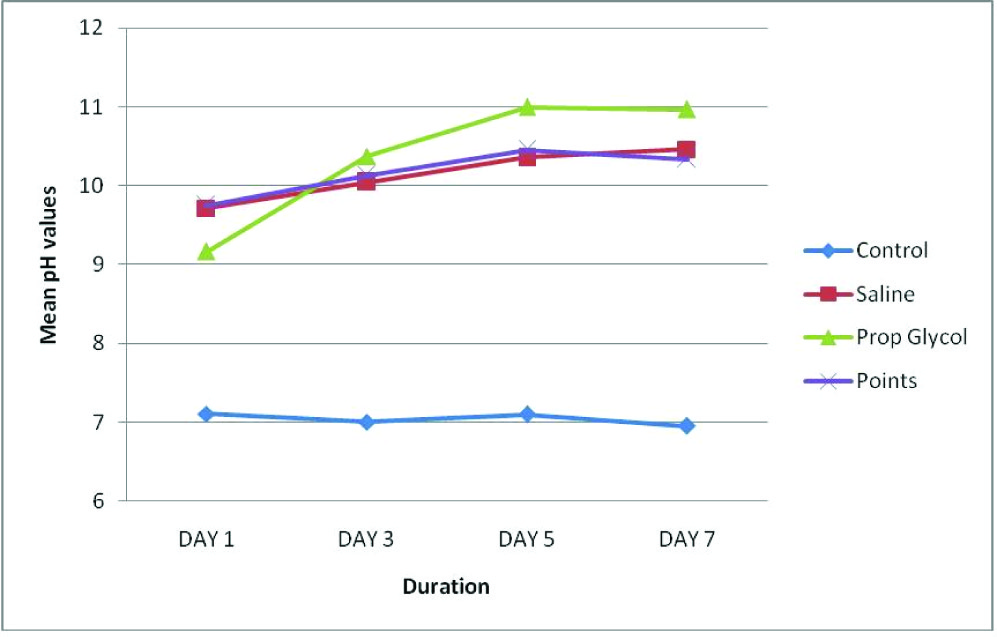
Mean pH values of all the groups and all the days over the observation period
| GROUP | Mean | N |
|---|
| DAY1 | Group-1 | 7.1093 | 15 |
| Group-2 | 9.7173 | 15 |
| Group-3 | 9.1656 | 15 |
| Group-4 | 9.7533 | 15 |
| Total | 8.9364 | 60 |
| DAY 3 | Group-1 | 7.0049 | 15 |
| Group-2 | 10.0451 | 15 |
| Group-3 | 10.3747 | 15 |
| Group-4 | 10.1294 | 15 |
| Total | 9.3885 | 60 |
| DAY 5 | Group-1 | 7.0971 | 15 |
| Group-2 | 10.3556 | 15 |
| Group-3 | 10.9940 | 15 |
| Group-4 | 10.4571 | 15 |
| Total | 9.7259 | 60 |
| DAY 7 | Group-1 | 6.9573 | 15 |
| Group-2 | 10.4602 | 15 |
| Group-3 | 10.9631 | 15 |
| Group-4 | 10.3358 | 15 |
| Total | 9.6791 | 60 |
Discussion
The use of medicaments has been a routine practice for many years as an adjunct to control bacterial contamination. They remain in the root canal between appointments thereby eliminate surviving bacteria [8].
In order to be an effective intracanal medicament, it should have a wide spectrum of activity, a reasonable duration of action, and should be in contact with the residual bacteria in sufficient concentration [9].
Calcium hydroxide has emerged as a popular intracanal medicament and is widely used in endodontics. Various biological properties have been attributed to this substance such as antimicrobial activity, tissue dissolving ability, inhibition of tooth resorption and induction of repair by hard tissue formaton [2]. The role of it’s high pH, ionic activity in the healing process and diffusion through dentinal tubules are aspects about this material which are still speculated.
In the present study two vehicles, saline (aqueous) and propylene glycol (viscous) were compared against Ca(OH)2 containing guttapercha points over a period of 7 d [10–12].
The first vehicle was an aqueous vehicle-Saline, saline was mixed with Ca(OH)2 powder manually to get a paste. Various studies have proved that Ca(OH)2 has high solubility and high alkalinity in aqueous solutions like saline, ringer’s solution and anesthetic solution [13–15].
The second vehicle was a viscous vehicle – Propylene glycol. Propylene glycol too was mixed with Ca(OH)2 powder manually to get a paste.
Propylene glycol has demonstrated a strong antibacterial action, it’s hygroscopic nature along with its high molecular weight (76.09) makes it an ideal vehicle for pharmaceutical preparations and has no toxic effects on tissues [5,16]. It has demonstrated a strong antibacterial action against common microorganisms found in infected root canals when used in concentrations upto 20% [17], in the present study 10% propylene glycol was used.
A innovation as a Calcium hydroxide carrier are specially prepared gutta percha points that contain Calcium hydroxide (52%) and are recommended as an intracanal medicament prior to obturation. They have claimed to overcome the poor handling properties and incomplete removal of calcium hydroxide suspensions. Therefore, this material was used in the present study [10,18,19].
As the diffusion is not constant in dentin an attempt was made to standardize the dentinal surface by creating a defect at the coronal third of the root. Hence, in the present study external defects measuring 3mm in diameter and 1mm in depth were prepared on the mesial surface in the coronal third of the roots [7,20,21]. This gave a uniform surface area of exposed dentinal tubules on the root for diffusion of the medicament. It also minimized the gross variability of surface area differences within the samples.
In the present study the calcium hydroxide points and saline groups exhibited the highest pH at day 1. The initial high pH value of the calcium hydroxide points may be due to the increased amount of Ca(OH)2 – 51-52% in it’s composition and the ability of the material to leach out in the moist environment of the root canal [19].
The initial high pH in the saline group may be due to the ability of saline to promote a high degree of solubility of the Ca(OH)2 powder. Another reason for the initial rise of pH, and later drop may be due to the formation of insoluble calcium carbonate crust which blocks the dentinal tubules resulting in decrease in the pH and stabilization of ionic release [22].
At day’s 3, 5 and 7 the maximum pH was recorded by the propylene glycol group, however the pH recorded was the highest at day 5 amongst all the days. At day 7, the pH of all the groups had dropped, with propylene glycol recording the maximum pH followed by saline and lastly calcium hydroxide points.
The high pH of the propylene group may be attributed to its high molecular weight (76.09) hygroscopic nature and viscosity thereby having sustained release of ions.
Thus, within the parameters of the in vitro study a viscous calcium hydroxide paste – propylene glycol remains the material of choice as a vehicle over calcium hydroxide points and aqueous (saline) calcium hydroxide paste. However, in applying the findings of an in vitro study to a clinical situation, it is important to realize in the oral environment there are many factors such as - temperature, extra cellular fluid, buffering capacity of dentin that have to be considered and cannot be mimicked in vitro studies. Therefore, further in vivo studies have to be conducted.
Conclusion
The following conclusions were drawn within the limitations of this in vitro study-
During the observation period, diffusion of ions had taken place and an alkaline pH was maintained by all the Calcium hydroxide formulations.
At day 1, a high pH was recorded by the Calcium hydroxide points and Saline groups and a lower pH by the Propylene glycol group.
At day’s 3 and 5, the maximum pH was recorded by the Propylene glycol group and minimum by the Calcium hydroxide points and Saline groups, however the pH recorded was the highest at day 5 amongst all the days.
At day 7, the pH of all the groups had dropped, with Propylene glycol recording the maximum pH followed by saline and lastly Calcium hydroxide points.