Fine Needle Aspiration Cytology of Male Breast Lesions – A Retrospective Study Over a Six Year Period
Kirana Pailoor1, Hilda Fernandes2, Jayaprakash CS3, Nisha J Marla4, Murali Keshava S5
1Associate Professor, Department of Pathology, Father Muller Medical College, Mangalore, India.
2Professor and Head, Department of Pathology, Father Muller Medical College, Mangalore, India.
3Professor, Department of Pathology, Father Muller Medical College, Mangalore, India.
4Associate Professor, Department of Pathology, Father Muller Medical College, Mangalore, India.
5Assistant Professor, Department of Pediatrics, Kasturba Medical College, Mangalore, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Kirana Pailoor ‘Omkara’, 15-17-902/3, 5th Cross,Shivbagh-Kadri, Mangalore, Karnataka, India. Phone : 9448953716,
E-mail: dockirana@yahoo.co.uk
Background: Fine needle aspiration cytology (FNAC) has a well-established role in the management of palpable breast lumps. However breast masses in males are rarely aspirated and hence there is limited cytopathologic experience. The aim of our study was to determine the efficacy of FNAC in the diagnosis of male breast lesions and also we attempted to describe the cytomorphological features of some of these lesions.
Materials and Methods: Data on male breast FNAC done between 2008 to 2013 were retrieved from the records of the cytopathology laboratory. FNAC diagnosis were categorized as benign, malignant, suspicious for malignancy and inadequate or unsatisfactory. Cytohistologic correlation was done with data from histopathology records. Sensitivity, specificity and diagnostic accuracy were calculated using standard statistical methods.
Results: Forty out of 1098 patients undergoing breast FNAC were males. Histopathology was available in 8 (20%) out of 40 cases. There were no false positive or false negative diagnoses. FNAC had a sensitivity, specificity and diagnostic accuracy of 100% for male breast lesions.
Conclusion: FNAC is a very accurate tool for the diagnosis of male breast lesions. It is highly sensitive and specific with good cytohistologic correlation. To reduce the high rate of surgical biopsies of benign male breast masses, we conclude that FNAC should be performed as a standard procedure in the clinical evaluation of male breast lesions
Breast carcinoma, Gynecomastia, Male breast lesions
Introduction
Fine needle aspiration cytology (FNAC) is a quick, accurate and cost-effective method in the workup of swellings from a variety of body sites. It is commonly used in the management and diagnosis of female breast lesions, but is seldom used in men, mainly because breast masses are less frequent in males. Gynecomastia is the most common cause of benign masses in the male breast. Carcinoma of the male breast is a rare disease representing 1% of all breast cancers and less than 1% of all cancer in men [1-12]. The aim of our study was to determine the efficacy of FNAC in the diagnosis of male breast lesions and also we attempted to describe the cytomorphologic features of a few of the lesions encountered in our series.
Materials and Methods
The medical records of all the patients who underwent FNAC of breast lumps at our centre between 2008 to 2013 were reviewed and data on the male breast aspirates were analysed. All aspirates were performed in the outpatient department using 23-gauge needle and five ml syringe by the cytopathologist. Air dried smears were prepared and stained by the May-Grunwald-Giemsa method. In addition, smears were wet-fixed in 95% ethyl alcohol and subsequently stained with Papanicolaou stain. The stained smears were classified into four major diagnostic categories such as benign, malignant, suspicious of malignancy and unsatisfactory aspirate. Histopathologic diagnosis was obtained wherever available and the cytologic diagnosis was retrospectively correlated with histologic findings. Finally statistical analysis was done by calculating the sensitivity, specificity and diagnostic accuracy of the aspirates.
Results
Over a six year period, 1098 patients with palpable breast lumps underwent Fine needle aspiration (FNA) at our hospital. Of these, 40 were males and out of which, 37 male patients had a unilateral FNA (left breast 19 and right breast 18) and three patients had a bilateral FNA [Table/Fig-1,2]. The age ranged from 18 to 85 yrs with a median age of 51.5 y. Diagnostic aspirates were obtained in all 40 cases. This was probably because the unstained smears were screened and checked immediately for the presence of material. These aspirates were each categorized into the following groups: benign/ reactive 36 (90%), malignant 1 (2.5%), atypical/suspicious for malignancy 3 (7.5%) and nondiagnostic/unsatisfactory 0(0%). The specificity, sensitivity and diagnostic accuracy was 100% in our study. This was probably due to the immediate screening of all the slides soon after the FNA procedure in every case.
Cytomorphologic features of the lesions in our series
The most common diagnostic entity encountered in our study was gynecomastia. Smears showed variable amount of cellular material, ranging from moderately cellular smears to extreme hypercellularity with numerous crowded tissue fragments. However, more commonly a moderately cellular smear pattern was noted. Smears showed large, tightly cohesive epithelial fragments often appearing as flat monolayered sheets. Mixed biphasic population of epithelial and stromal fragments were also seen [Table/Fig-3]. Scattered bipolar to oval myoepithelial nuclei were seen in the background of the smears. Only eight cases were confirmed histologically [Table/Fig-4]. We had one case of infiltrating ductal carcinoma which was hypercellular with tumour cells in discohesive sheets and dispersed singly. These cells had pleomorphic vesicular nuclei with prominent nucleoli and had abundant eosinophilic cytoplasm [Table/Fig-5]. Histologically, it was diagnosed to be Oncocytic carcinoma [Table/Fig-6]. Cohesive sheets of benign ductal epithelial cells, a few cyst macrophages and sheets of apocrine cells were observed in a case of fibrocystic disease [Table/Fig-7]. Diffuse sheets of inflammatory cells composed predominantly of neutrophils admixed with lymphocytes and histiocytes were seen amidst occasional sheets of ductal epithelial cells in a case of breast abscess [Table/Fig-8].
Discussion
Breast masses in males constitute less than 2% of the total cases in large FNAC studies of breast lumps. Carcinoma in male breast is very rare as compared to the female breast [1-12]. In our series, the total number of patients who underwent FNAC for the assessment of a breast lump was 1098 over a six year period with males constituting 3.6% (40 out of 1098). This was almost similar to a study by MacIntosh et al., [13] (3.2%). However, Westend [14] and Wauters et al., [15] had very few cases such as 1.5% and 1.7% respectively. Martin Bates [16] and Russin et al., [17] found a near-total unilateral involvement in Gynecomastia similar to the present study. In our study, 30 out of 40 cases (75%) were Gynecomastia and 11 out of 30 cases (37%) of them presented as subareolar mass. Gynecomastia was unilateral in 90% of the cases (27 out of 30) and more frequent in the left than right side (15 cases were left sided and 12 were in right sided). This was similar to the studies conducted by Das et al., [18] and Martin-Bates et al., [16] who observed it more in the left breast.
The age at presentation was variable with the greatest prevalence in the third decade. The peak age was fifth decade in our study. Russin et al.,[17]observed bimodal peak in the third and seventh decades. The age at detection of breast carcinoma in males averages just under 60 y, which is six to eleven years later than the average age in women. We had only one case of male breast carcinoma that was 55 y old. This was in contrast to other studies done by Siddiqui [19], Westend [14], MacIntosh [13] and Wauters et al.,[15] who had more number of cases [Table/Fig-9].
Gynecomastia is the most common cause of masses in the male breast and is defined as the enlargement of the male breast due to proliferation of both glandular and stromal elements. The FNAC features of gynecomastia include three components such as cell-poor to cell-moderate, cohesive sheets or groups of bland cells, bipolar bare nuclei and single, tall columnar cells. Mild to moderate cellularity was observed in 86% of cases by Russin and associates [17], in 96.2
% of cases by Das et al., [18] and in almost all cases (100%) in the present study. However, Gupta et al.,[20] observed rich cellularity bin 79.1% of cases. Mild nuclear atypia was observed in three cases (7.5%) in our study whereas it was seen in 5.3% and 9.3% of cases in Das et al and Gupta et al respectively
There was only one case of Infiltrating ductal carcinoma on FNA (2.5%) which was subsequently diagnosed to be Oncocytic carcinoma on histopathology in our study. This case was cytologically distinct from Gynecomastia. High cellularity, dyshesive cell groups and anisonucleosis was observed. These tumour cells had abundant cytoplasm. Siddiqui et al.,[19] also had 2.8% of malignant cases which is almost similar to our study. However, Westend et al.,[14] , MacIntosh et al.,[13] and Wauters et al.,[15] had 9.8%, 7.9% and 10.2% of the cases respectively.
In the present study, we had 20% ( 8 out of 40) cases with histologic follow-up which is similar to that of a study by MacIntosh et al.,[13] (17%) but in contrast to that of Westend et al.,[14] (47%) and Wauters et al.,[15] (58%). The number of unsatisfactory cases ranged from 11.7% to 33.3% in various studies. However, we did not have any, due to the immediate screening of unstained slides for material [Table/Fig-2]. We noticed 100% sensitivity similar to that of Westend et al., [14] and Wauters et al., [15] and 100% specificity similar to the studies by Siddiqui et al.,[19] and MacIntosh et al.,[13] .
Depicts the details of 20 male breast lesions
| Serial no. | Age (years) | Side | Lesion | FNA diagnosis | Histopathology diagnosis |
| 1 | 55 | Right | Subareolar mass | Gynecomastia | Not done |
| 2 | 65 | Left | Vague lumpiness | Fibrocystic disease | Not done |
| 3 | 52 | Right | Subareolar mass | Gynecomastia | Gynecomastia |
| 4 | 22 | Left | Subareolar mass | Gynecomastia | Not done |
| 5 | 18 | Right | Subareolar mass | Gynecomastia | Not done |
| 6 | 55 | Left | Vague lumpiness | Gynecomastia | Not done |
| 7 | 63 | Left | Nodule | Gynecomastia | Not done |
| 8 | 36 | Right | Nodule | Gynecomastia | Gynecomastia |
| 9 | 68 | Bilateral | Subareolar mass | Gynecomastia | Not done |
| 10 | 28 | Left | Nodule | Gynecomastia | Not done |
| 11 | 70 | Right | Vague lumpiness | Gynecomastia | Not done |
| 12 | 61 | Right | Subareolar mass | Gynecomastia | Not done |
| 13 | 51 | Right | Subareolar mass | Benign proliferative breast disease | Not done |
| 14 | 33 | Left | Vague lumpiness | Gynecomastia | Not done |
| 15 | 65 | Left | Vague lumpiness | Gynecomastia | Not done |
| 16 | 85 | Right | Vague lumpiness | Gynecomastia | Not done |
| 17 | 54 | Right | Firm lump | Breast abscess | Not done |
| 18 | 55 | Right | Hard,fixed lump | Infiltrating ductal carcinoma | Oncocytic Carcinoma |
| 19 | 78 | Left | Subareolar mass | Gynecomastia with atypia | Not done |
| 20 | 45 | Right | Vague lumpiness | Gynecomastia | Not done |
Depicts the details of another 20 male breast lesions
| Serial no. | Age (years) | Side | Lesion | FNA diagnosis | Histopathology diagnosis |
| 21 | 73 | Right | Nodule | Fibrocystic disease | Not done |
| 22 | 49 | Left | Vague lumpiness | Gynecomastiae | Not done |
| 23 | 51 | Left | Nodule | Gynecomastia | Not done |
| 24 | 54 | Right | Subareolar mass | Gynecomastia | Not done |
| 25 | 65 | Right | Nodule | Gynecomastia | Not done |
| 26 | 45 | Right | Vague mass | Fibrocystic disease | Not done |
| 27 | 63 | Left | Vague mass | Gynecomastia | Not done |
| 28 | 30 | Right | Subareolar mass | Gynecomastia | Gynecomastia |
| 29 | 22 | Left | Vague lumpiness | Gynecomastia with mild atypia | Gynecomastia |
| 30 | 51 | Left | Vague lumpiness | Gynecomastia | Not done |
| 31 | 41 | Left | Vague lumpiness | Gynecomastia | Not done |
| 32 | 31 | Bilateral | Vague lumpiness | Bilateral Gynecomastia | Not done |
| 33 | 35 | Left | Vague lumpiness | Gynecomastia | Gynecomastia |
| 34 | 19 | Right | Vague lumpiness | Gynecomastia | Not done |
| 35 | 43 | Left | Subareolar mass | Chronic mastitis | Not done |
| 36 | 35 | Left | Nodular enlargement | Gynecomastia | Not done |
| 37 | 21 | Bilateral | Vague lumpiness | Bilateral Gynecomastia | Not done |
| 38 | 28 | Left | Vague lumpiness | Gynecomastia | Gynecomastia |
| 39 | 38 | Right | Firm lump | Breast abscess | Not done |
| 40 | 45 | Left | Vague lumpiness | Gynecomastia | Not done |
Smear shows monolayered sheets of benign ductal epithelial cells and stromal fragments (Papanicolaou x 100X)
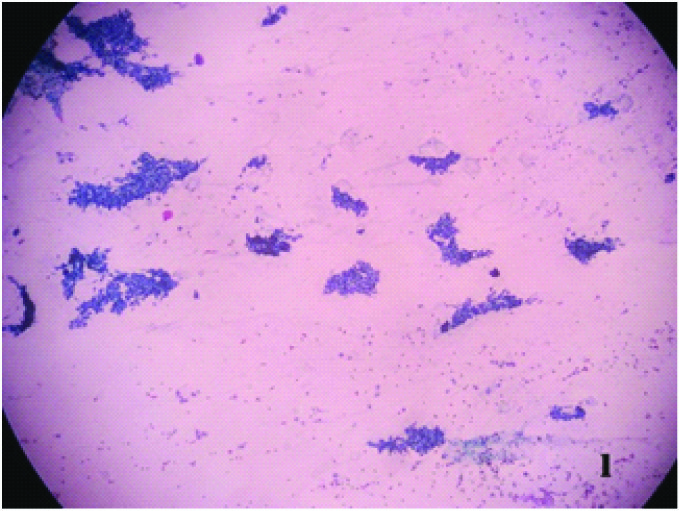
Histopathology section of the same which confirms Gynecomastia (H & E x100X)

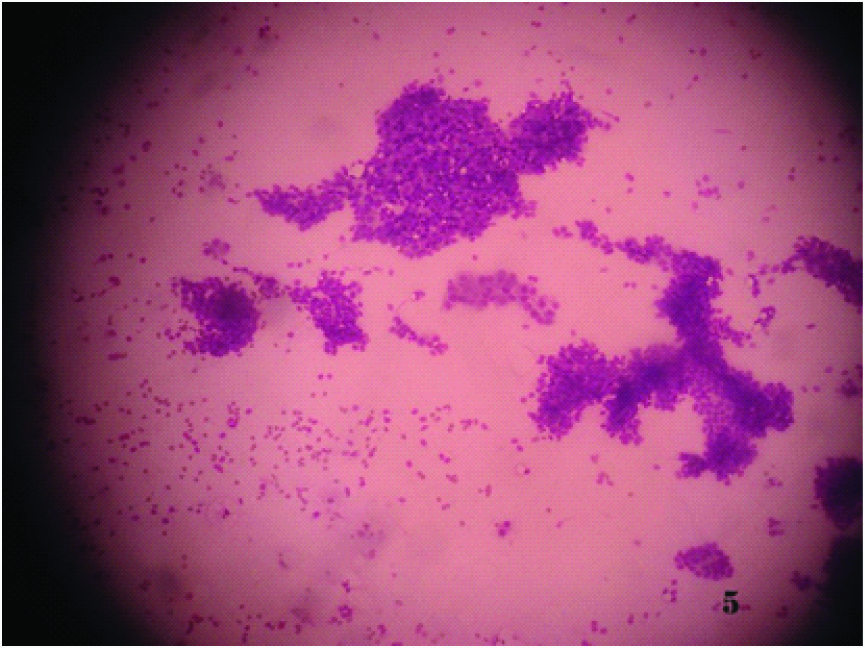
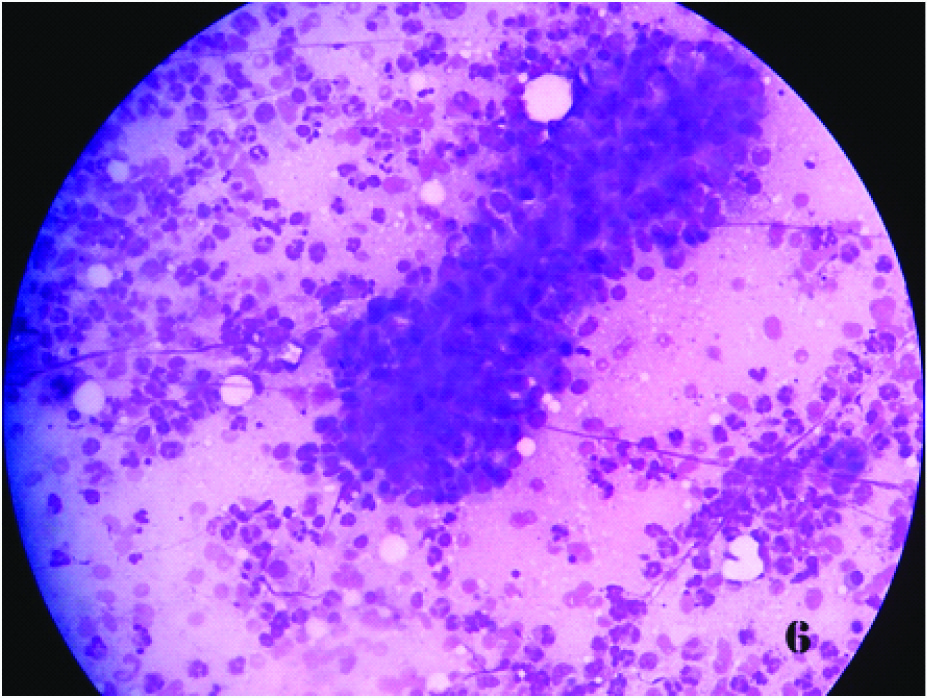
Smear shows singly dispersed tumour cells with abundant cytoplasm (Papnicolaou x 100X)
Section shows nests of tumour cells of the same case (H & Ex 100X)

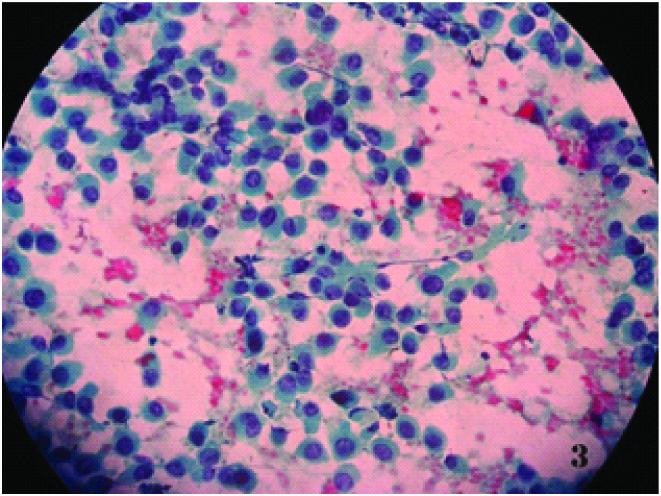
Smear shows benign ductal epithelial cells and apocrine cells in sheets ( May Grunwald Giemsa x 100X))
Smear shows benign ductal epithelial cells admixed with dense inflammatory cells ( May Grunwald Giemsa x100X)
Results of the present study in comparison with other studies
| Result | 2002 Westend et al., [14] | 2002 Siddiqui et al., [19] | 2008 MacIntosh et al., [13] | 2009 Wauters et al., [15] | 2014 present study |
| No. of male FNA | 153 (1.5%) | 614 (4.3%) | 138 (3.2%) | 147 (1.7%) | 40 (3.6%) |
| No. of cases with histologic follow up | 72 (47%) | 170 (28%) | 23 (17%) | 85 (58%) | 8 (20%) |
| No. of malignant cases | 15 (9.8%) | 26 (2.8%) | 11 (7.9%) | 15 (10.2%) | 1 (4.16%) |
| No.of unsatisfactory cases of all FNAs | 18 (11.7%) | 94 (15.4%) | 46 (33.3%) | 45 (30.6%) | 0 |
| Sensitivity | 100% | 95.3% | 95.5% | 100% | 100% |
| Specificity | 89% | 100% | 100% | 90.2% | 100% |
Conclusion
FNAC is a very sensitive and specific diagnostic tool for the assessment of breast masses in male patients. The routine use of FNAC and immediate screening of unstained slides for the presence of adequate material would greatly reduce the number of unnecessary biopsies and frozen sections for histopathologic evaluation, especially in case of Gynecomastia. Hence, we strongly recommend the use of FNAC as the first-line investigation in the clinical evaluation of male breast lumps.
[1]. DG Rosen, R Laucirica, G Verstovsek, Fine needle aspiration of male breast lesions.Acta Cytol 2009 53:369-74. [Google Scholar]
[2]. JF Silverman, DR Lannin, K O’Brien, HT Norris, The triage role of fine needle aspiration biopsy of palpable breast masses- diagnostic accuracy and costeffectiveness.Acta Cytol 1987 31(6):731-36. [Google Scholar]
[3]. L Palombini, F Fulciniti, A Vetrani, Fine needle aspiration biopsies of Breast masses – a critical analysis of 1956 cases in 8 years.Cancer 1988 61:2273-7. [Google Scholar]
[4]. R Lilleng, N Paksoy, G Vural, F Langmark, B Hagmar, Assesment of fine needle aspiration cytology and histopathology for diagnosing male breast masses.Acta Cytol 1995 39:877-81. [Google Scholar]
[5]. GE Feichter, F Haberthur, S Gobat, P Dalquen, Breast cytology- statistical analysis and cytohistologic correlations. Acta Cytol 1997 41:327-32. [Google Scholar]
[6]. A Joshi, K Kapila, K Verma, Fine needle aspiration cytology of male breast masses- nineteen years experience.Acta Cytol. 1999 43(1):334-38. [Google Scholar]
[7]. N Bhat, EF Rosato, PK Gupta, Gynecomastia in a Mortician.Acta Cytol 1990 34(1):31-34. [Google Scholar]
[8]. M Amrikachi, LK Green, R Rone, I Ramzy, Gynecomastia- Cytologic features and diagnostic pitfalls in fine needle aspirates.Acta Cytol 2001 45(6):948-52. [Google Scholar]
[9]. B Rai, S Ghoshal, SC Sharma, Breast cancer in males: A PGIMER experienceJ Can Res Ther 2005 1:31-33. [Google Scholar]
[10]. R Rone, I Ramzy, A Northcutt, Gynecomastia: Cytologic features and diagnostic pitfalls in asoiration biopsiesActa Cytol 1986 30(5):589 [Google Scholar]
[11]. S Coghill, A Howat, Inflammatory conditions and benign breast lesions. In: Diagnostic Cytopathology. Gray W, McKee GTElsevier 2004 2nd Edition:257-78. [Google Scholar]
[12]. JF Silverman, RS Saad, Breast. In: Comprehensive Cytopathology. Bibbo M, Wilbur D.Elsevier 2008 3rd Edition:713-773. [Google Scholar]
[13]. RF MacIntosh, JL Merrimen, PJ Barnes, Application of the probabilistic approach to reporting breast fine needle aspiration in males.Acta Cytol 2008 52:530-34. [Google Scholar]
[14]. PJ Westend, C Jobse, Evaluation of fine-needle aspiration cytology of breast masses in males.Cancer (Cancer Cytopathol) 2002 96:101-04. [Google Scholar]
[15]. CAP Wauters, BW Kooistra, IMK Heijden, LJA Strobbe, Is cytology useful in the diagnostic workup of male breast lesions? A retrospective study over a 16-year period and review of the recent literature.Acta Cytol 2010 54:259-64. [Google Scholar]
[16]. E Martin-Bates, T Krausz, I Phillips, Evaluation of fine needle aspiration of the male breast for the diagnosis of gynecomastiaCytopathol. 1990 1:79-85. [Google Scholar]
[17]. VL Russin, C Lachowicz, TS Kline, Male breast lesions: Gynecomastia and its distinction from carcinoma by aspiration biopsy cytologyDiagn Cytopathol 1989 5:243-47. [Google Scholar]
[18]. DK Das, TA Junaid, SB Mathews, Fine needle aspiration cytology diagnosis of male breast lesions – a study of 185 cases.Acta Cytol 1995 39:870-76. [Google Scholar]
[19]. MT Siddiqui, MF Zakowski, R Ashfaq, SZ Ali, Breast masses in males: Multiinstitutional experience on fine needle aspiration.Diagn Cytopathol 2002 26:87-91. [Google Scholar]
[20]. RK Gupta, S Naran, J Simpson, The role of fine needle aspiration cytology in the diagnosis of breast masses in malesEur J Surg Oncol 1988 14:317-20. [Google Scholar]