Premature Ejaculation – Dose and Duration Dependent Effect of Fluoxetine: A Histological Study on Seminal Vesicle of Albino Rats
Alka Aggarwal1, SL Jethani2, RK Rohatagi2, Juhi Kalra2
1 Assistant Professor, Department of Anatomy, Himalayan Institute of Medical Sciences, SRHU, Dehradun, U.K. India.
2 Professor and Head, Department of Anatomy, Himalayan Institute of Medical Sciences, SRHU, Dehradun, U.K. India.
3 Professor, Department of Anatomy, Himalayan Institute of Medical Sciences, SRHU, Dehradun, U.K. India.
4 Assosiate Professor, Department of Pharmacology, Himalayan Institute of Medical Sciences, SRHU, Dehradun, U.K. India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Alka Aggarwal, Assistant Professor, Department of Anatomy, Himalayan Institute of Medical Sciences, Swami Rama Himalayan University (SRHU), Dehradun, U.K. India. E-mail : alkadr2011@rediffmail.com
Introduction: Fluoxetine is a prototype drug of Selective Serotonin Reuptake Inhibitors. Its active demethylated metabolite has a half life of 7-10 d. Fluoxetine is used to treat depression and is also prescribed in premature ejaculation.
Aim: In the present study dose and duration dependent effects of Fluoxetine on histology of seminal vesicle of the albino rats were observed.
Materials and Methods: The present study was conducted on 36 adult male albino rats. Fluoxetine was administered intraperitoneally for 2 wk, 4 wk and 12 wk with mild (10mg/kg/day), moderate (20mg/kg/day) and severe doses (40mg/kg/day). Histological slides of Seminal vesicle were prepared and stained with Hematoxylin and Eosin stain.
Results: On examination through the light microscope, the proliferation of primary, secondary and tertiary villi, increased crypt/alveoli, increased thickness of lamina propria, decreased epithelial cell height, metaplasia, changes in the amount of luminal eosinophilic secretory material in the form of scanty secretion in lumen of seminal vesicle.
Conclusion: Low doses for long duration and high doses for short duration of Fluoxetine produce histological changes in seminal vesicle of albino rats.
Delayed ejaculation, 5-hydroxytryptamine, Male fertility, Metaplasia, Selective serotonin reuptake inhibitors
Introduction
The relative safety and better acceptability of Selective Serotonin Reuptake Inhibitors (SSRIs) have made them first line antidepressant drugs. Fluoxetine is prototype of the SSRIs and longest acting, with a plasma half life of 2 d. Its active demethylated metabolite has a half life of 7-10 d [1].
Fluoxetine inhibits the 5–hydroxytryptamine (5-HT) (Serotonin) reuptake. Increased synaptic availability of serotonin stimulates a large number of postsynaptic (5-HT) receptor subtypes (5-HT1A, 5-HT1B, 5-HT2C) which lead to sexual adverse effects of SSRIs such as loss of libido, delayed ejaculation, anorgasmia and impaired orgasm [2,3]. Studies showed that serum 5-HT level may be used as a functional diagnostic tool for premature ejaculation (PE) and an indicator in PE treatment [4].
SSRIs induced delayed ejaculation in humans has given encouraging results when used in the treatment of PE [5,6]. Clinical studies showed that intravaginal ejaculation latency time (IELT) after treatment with SSRIs gradually increased from 18 sec to approximately 170 sec [7].
The aim of the present study was to investigate the effects of varying doses for different durations of fluoxetine administration on the histology of seminal vesicle which is used in the treatment of PE. There is hardly any reported literature on fluoxetine induced histological changes on seminal vesicle. This also has been an investigating factor to carry out this experimental study.
Materials and Methods
The study was done on 36 male Albino rats (Rattus norwegicus, Wistar strain) with an average weight of 120-150 g. These animals were obtained from the Central Animal House of Himalayan Institute of Medical Sciences, Dehradun, after obtaining the prior approval of Institutional Animal Ethical Committee (IAEC). Before starting the study it was confirmed that all the rats were disease-free and healthy. All the groups of rats were housed separately in different cages in groups of 2 to 3 rats per cage. They were fed with standard rodent pellet diet and water was allowed ad libitum. After 2wk of acclimatization period in individual cages under a 12Light: 12Dark cycle this study was started.
This study was conducted in 3 Phases of 2, 4 and 12 wk duration. Each phase comprised of 12 rats. These 12 rats were further subdivided into 4 groups (one Control and three Experimental groups) of 3 rats each. Group 1(Control) rats received equal volume of normal saline (vehicle) intraperitoneally. The Experimental groups (Group 2, Group 3 & Group 4) rats were injected with 10 mg/kg/day, 20 mg/kg/day and 40 mg/kg/day of fluoxetine intra-peritoneal respectively. After completion of each of the three phases, the seminal vesicle was dissected out after sacrificing all the rats under ether anaesthesia. The tissues were fixed in 10% formalin, processed and blocks were made in paraffin wax. 4-5 μm thick sections were cut and stained with hematoxylin and eosin (H&E) stain. The sections were examined in the light microscope under magnification (200X, 400X). Ten randomly selected fields were taken from each control and experimental group sections following which the mean and standard deviations were calculated. The Morphometric analysis was done under 400X magnification with the help of eyepiece micrometer. The nucleuses of cell were counted to count the number of cells per 10 micrometer. Student’s t-test was used for statistical analysis.
Results
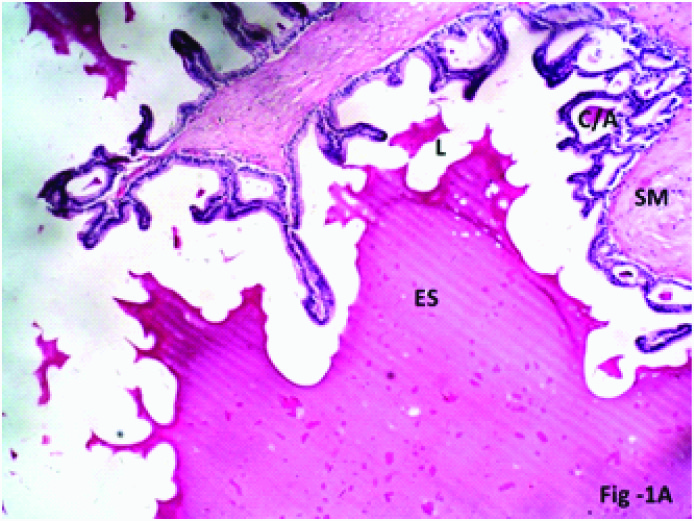
The transverse section (T.S.) of seminal vesicle of Group 1 (Control) showed mucosal folds/ridges extending into the lumen. The lining epithelium of seminal vesicle was Pseudo-stratified columnar epithelium, composed mainly of a single layer of tall columnar principal cells and triangular shaped basal cells. The lumen of seminal vesicle was filled with eosinophilic secretion. The epithelium lies on a thin layer of connective tissue lamina propria. The lamina propria was lined by inner circular and outer longitudinal smooth muscle fibres. Mucosal alveoli/crypts showed small amount of secretion [Table/Fig-1a,b].
Photomicrograph of the seminal vesicle in Group 1 (Control) of albino rats showing normal pseudostratified columnar epithelium (E), smooth Muscle (SM) lumen (L) filled with eosinophilic secretion (ES), few mucosal crypts/alveoli (C/A) H & E; X 200
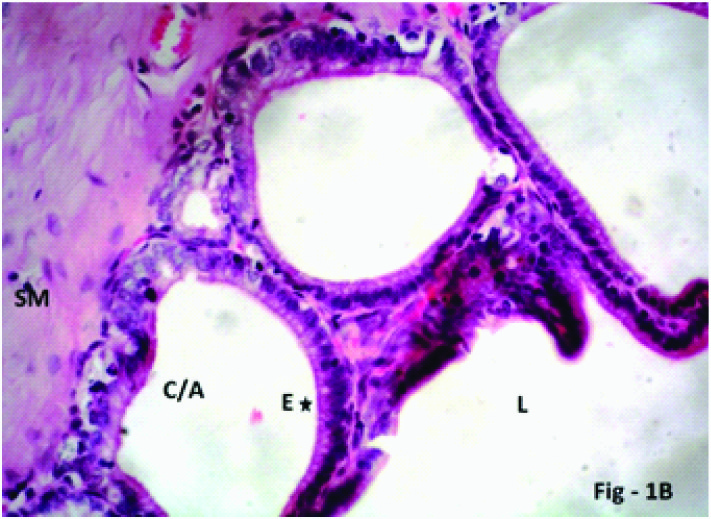
Photomicrograph of the seminal vesicle in Group 1 (Control) of albino rats showing mucosal crypt/alveolus (C/A) lined by normal pseudostratified columnar epithelium (E, five point star) H & E; X 400
It was observed that Experimental rats (Group 2) which were administered Fluoxetine (10mg/kg/day) showed a normal histological appearance of seminal vesicle in phase I & II. Phase III rats showed reduction in lumen size due to proliferation of mucosal folds. The number of crypt/alveoli increased with scanty eosinophilic secretion. The epithelial cell height decreased at some places and stratification of epithelial cells also seen at some places [Table/Fig-2,3and4a,b].
Changes in the mean number of cells/10μ length of epithelium of Seminal vesicle
| Groups | 2 weeks (Mean ± SD) | 4 weeks (Mean ± SD) | 12 weeks (Mean ± SD) |
|---|
| Group1 (Control) | 8.00 ± 0.32 | 8.20 ± 0.30 | 7.70 ± 0.30 |
| Group 2 (10mg/kg/day) | 8.20 ± 0.00 p=0.172 | 7.90 ± 0.42 p=0.172 | 8.00 ±0.00 p=0.1717 |
| Group 3 (20mg/kg/day) | 7.70 ± 0.47 p=0.1717 | 8.00 ± 0.00 p=0.1717 | 8.00 ± 0.00 p=0.1717 |
| Group 4 (40mg/kg/day) | 7.70 ± 0.47 p=0.296 | - | - |
(p < 0.01 – Highly significant, p < 0.05 – Significant, p > 0.05 – Non significant)
Changes in the mean height of Seminal vesicle epithelial cells
| Groups | 2 weeks (Mean ± SD) | 4 weeks (Mean ± SD) | 12 weeks (Mean ± SD) |
|---|
| Group1 (Control) | 3.10 ± 0.32 | 3.0 ± 0.00 | 3 ± 0.00 |
| Group 2 (10mg/kg/day) | 3.20 ± 0.42 p=0.296 | 2.80 ± 0.42 p=0.084 | 2.40 ± 0.49 p=0.00256 |
| Group 3 (20mg/kg/day) | 2.9 ± 0.32 p=0.0839 | 1.90 ± 0.42 p=0.00000008 | 1.20 ± 0.40 p=0.0000004 |
| Group 4 (40mg/kg/day) | 1.70 ± 0.48 p=0.001 | - | - |
(p < 0.01 – Highly significant, p < 0.05 – Significant, p > 0.05 – Non significant)
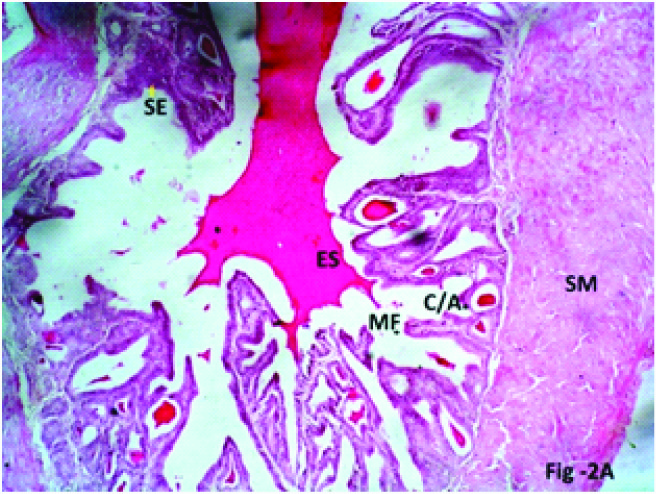
Photomicrograph of the seminal vesicle in Phase III (12 weeks), Group 2 (10mg/kg/day) experimental albino rats showing complex mucosal folds (MF, four point star), with formation of mucosal crypts/alveoli (C/A, five point star), eosinophilic secretion (ES) within the crypts and lumen, stratification of epithelial cells (SE, yellow arrow) H & E; X 200
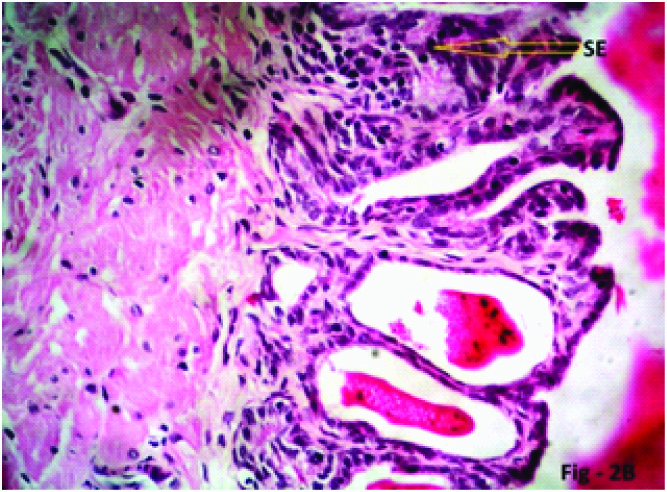
Photomicrograph of the seminal vesicle in Phase III (12 weeks), Group 2 (10mg/kg/day) experimental albino rats showing stratification of mucosal epithelial cells (SE, yellow arrow) H & E; X 400
Group 3 Experimental rats (20mg/kg/day) phase I also showed a normal histological appearance but epithelial cell height decreased. In Phase II mucosal folds/ridges completely divided the lumen into compartments. The height of epithelial cells decreased. In Phase III mucosal folds reached upto the centre with increased number of mucosal crypt/alveoli. Mucosal crypts were filled with small amount of secretion. Epithelial cell height decreased further. In some fields the cells became flattened while in other places stratification of cells was found [Table/Fig-2,3,5a,b].
Photomicrograph of the seminal vesicle in Phase III (12weeks), Group 3 (20 mg/kg/day) experimental rats showing Lumen filled with mucosal crypts/alveoli (C/A) with scanty eosinophilic secretion (ES), Lamina propria penetrating within the mucosal folds H & E; X 200
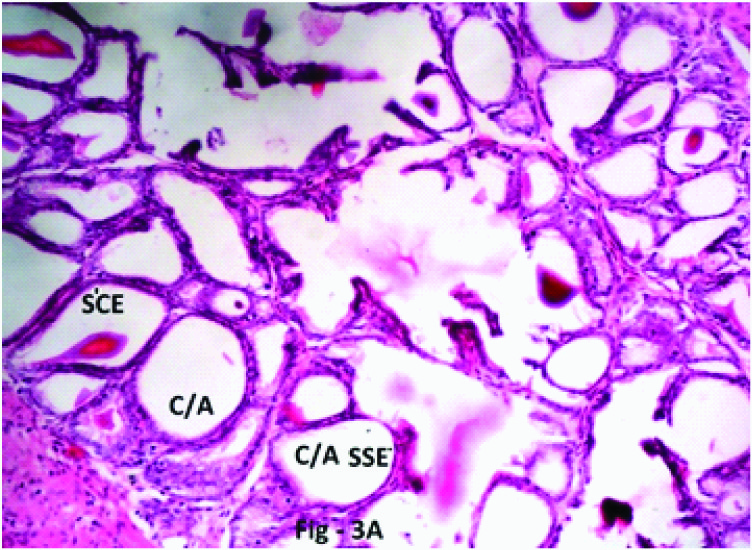
photomicrograph of the Seminal vesicle in Phase III (12 weeks), Group 3 (20 mg/kg/day) Crypt/Alveoli (C/A) lined by simple squamous epithelium (SSE)/Simple cuboidal epithelium (SCE) H & E; X 400
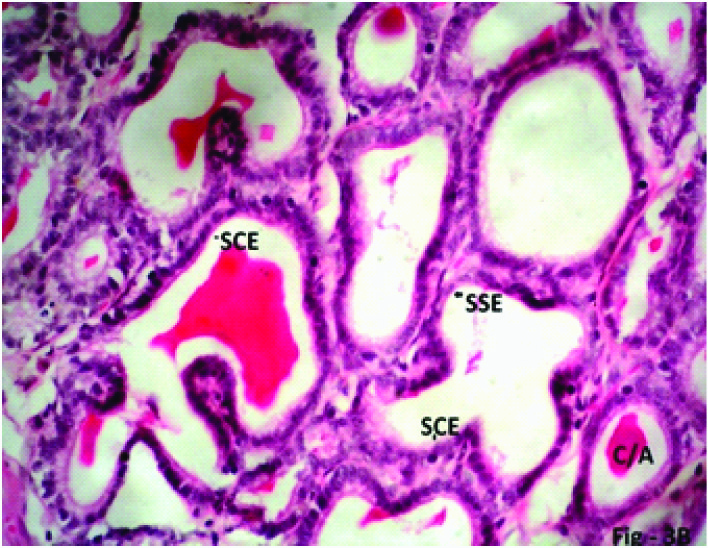
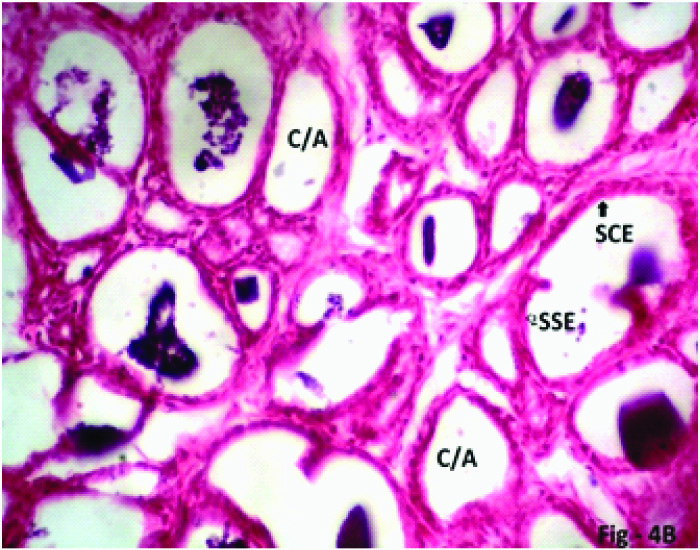
Some Experimental rats of Group 4 (40mg/kg/day) which were sacrificed on 7th day due to ill health showed that grossly the seminal vesicle decreased in size. The diameter of seminal vesicle also decreased. The height of epithelial cells decreased (become flattened) at some places. In other places stratification of cells found. Mucosal folding increased with increased crypts/alveoli. The connective tissue (lamina propria) increased in amount and was found creeping within the folds of epithelium [Table/Fig-2,3,6a,b].
Photomicrograph of the seminal vesicle in Phase I (2weeks), Group 4 (40mg/kg/day) experimental rats showing mucosal folds completely obliterating the lumen. Stratification of epithelial cells (SE), Lamina propria (LP) penetrating in the mucosal folds, plenty of mucosal crypts/alveoli (C/A) lined by simple squamous epithelium (SSE, arrow) and simple cuboidal epithelium (SCE, arrow) with scanty eosinophilic secretion (ES) H & E; X 200
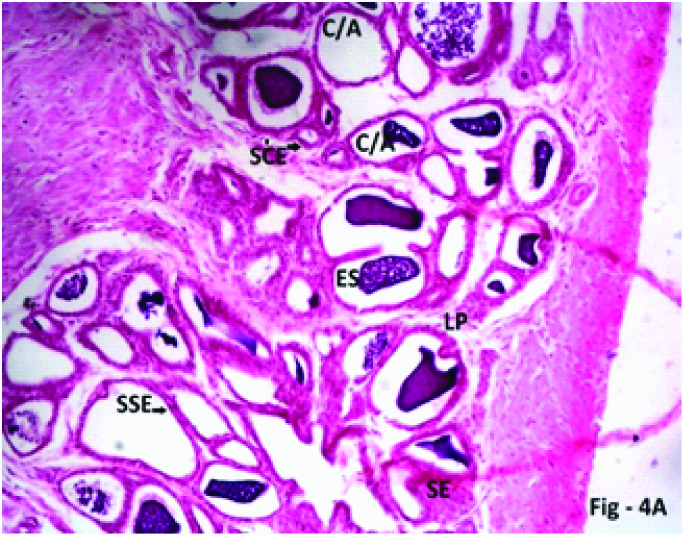
Photomicrograph of the seminal vesicle in Phase I (2weeks) Group 4 (40mg/kg/day) experimental rats showing Crypts/Alveoli (C/A) lined by simple cuboidal epithelium (SCE, black arrow), simple squamous epithelium (SSE, black triangle) H & E; X 400
Discussion
The seminal vesicles are elongated saccular organs with numerous lateral outpocketings forming an irregularly branched lumen. The wall consists of an external connective tissue layer rich in elastic fibres, a middle layer of smooth muscle and an epithelium resting upon a layer of loose connective tissue. The mucosa forms primary folds, which branch into secondary and tertiary folds. These project into the lumen and anastomose frequently and form numerous crypts of different sizes, separated by thin branching partitions. All of these crypts open into central lumen. The epithelium is pseudo stratified and consists of rounded basal cells lodged between larger cuboidal or low columnar cells. The secretion of seminal vesicle is a slightly yellowish, viscid liquid. Seminal vesicles mainly secrete prostaglandins and fructose. It appears as coagulated, deeply staining masses in the lumen. Testosterone is essential for the maintenance of height of the mucosal epithelium. It also influences the function of smooth muscle in the seminal vesicles [8,9]. The height of the cells often reflects the level of secretory or absorptive activity. Epithelia involved in secretion or absorption are simple columnar or in a few cases pseudo stratified [10]. In the present study fluoxetine treated rats in Phase III of Group 2, 3 & Phase I of Group 4 showed epithelial metaplasia in the form of low columnar, cuboidal, and simple squamous and stratified squamous cells. This epithelial metaplasia cause scanty secretion and proliferation of mucosal folds upto the centre of lumen causing the narrowing of lumen which may be helpful in prolonging the duration of onset of ejaculation in PE subjects. These changes may cause delay in ejaculation.
Studies showed that after treatment with Fluoxetine, men experience fertility related problems in the form of poor semen quality such as decreased sperm viability and sperm motility [11]. These problems have been proposed to be related to the accessory sex glands dysfunction. The secretion of the seminal vesicles contains fructose, other simple sugars, amino acids, ascorbic acid and prostaglandins. Fructose is the primary nutrient source for the sperms in the semen. Seminal vesicular secretion is important for semen coagulation, sperm motility, and stability of sperm chromatin and suppression of the immune activity in the female reproductive tract [11–13]. The mucosal changes found in the present study leads to hypofunction of the seminal vesicles.
Studies showed that the change in testosterone only affects the epithelial cells in the form of proliferation but stromal cells remained unaffected. Testosterone decreases the level of basic secretory proteins in the seminal vesicles [14,15]. In the present study proliferation of mucosal folds along with the stromal cells were found. Previous studies have reported serotonergic neural fibers in seminal vesicles in different species [15]. In rat seminal vesicle 5-HT1A, -1B and -2c receptor subtypes were found and were responsible for the peripheral role of 5-HT in the regulation of contractile responses of the seminal tract [16].
PE can be defined by ≤1-min ejaculatory latency, an inability to delay ejaculation. PE occurs in as many as 40% of men. Contraction of the smooth muscle coat during ejaculation forces the secretions of the seminal vesicles into the ejaculatory ducts [3]. In Present study thickness of smooth muscle increased as the duration and dose of fluoxetine increased.
Ejaculatory disorders are classified into 3 main groups. These groups are unsatisfactory timing of ejaculation, including PE; retarded (delayed) ejaculation; and absence of ejaculation, including partial or complete inability to ejaculate [3]. Fluoxetine is one of the drugs which have been reported to retard testicular development and decrease in the number of Leydig cells, sertoli cell and germinal cell series in male rats [17]. These changes lead to decreased testosterone which leads to decrease seminal vesicle weight and secretions [14,15]. In the present study the Group 4 rats which received high doses (40mg/kg/day) of the drug showed decreased weight of the seminal vesicles. Hence, in present study this change could be attributable to effect of fluoxetine on hormonal axis affecting testosterone level.
Clinical studies have shown that following treatment with SSRIs the patients developed complaints of absence of ejaculation, partial or complete inability of ejaculation [18]. The cause may be the scanty secretion in the seminal vesicles due to squamous metaplasia or narrowing of lumen due to proliferation of mucosal folds.
Conclusion
Since SSRIs are frequently prescribed by physicians in high doses and for long duration for various psychiatric disorders and even misused by the peoples. Present study showed that long duration low doses and short duration high doses Fluoxetine produces such histological changes in seminal vesicle which may impair sexual function. Sexual side effects produced by SSRIs affect the quality of life and may result in non-compliance with medication and associated risk of re-occurrence of depression. It is a necessity to further investigate for safe doses and safe duration of SSRIs & also to access reversibility of these histological changes in the seminal vesicle.
(p < 0.01 – Highly significant, p < 0.05 – Significant, p > 0.05 – Non significant)
(p < 0.01 – Highly significant, p < 0.05 – Significant, p > 0.05 – Non significant)
[1]. Tripathi KD, Drugs used in mental illness: Antidepressants and Antianxiety Drugs. In: Tripathi KD, Tripathi M, edEssentials of Medical Pharmacology 2008 6th edNew Delhi (India)JayPee Brothers Medical Publishers (P) Ltd [Google Scholar]
[2]. Brunton L, Bruce AC, Björn CK, Section II Neuropharmacology Drug therapy of Depression and anxiety Disorders. In: Laurence LB, edGoodman and Gillman’s The Pharmacological Basis of Therapeutics 2011 12th editionNew YorkMc Graw-Hill Companies [Google Scholar]
[3]. François G, Pierre C, Physiology of Ejaculation: Emphasis on Serotonergic ControlEuropean Journal 2005 (http://www.europeanurology.com/article/S0302-2838 (05)00310-6) [Google Scholar]
[4]. Yang C, Tang K, Wang B, Clinical Value of Serum 5-HT Level in Diagnosis and Treatment of Premature EjaculationUrol Int 2013 90:214-18. [Google Scholar]
[5]. Waldinger MD, Olivier B, Utility of selective serotonin reuptake inhibitors in premature ejaculationCurr Opin Investig Drugs 2004 5:743-47. [Google Scholar]
[6]. Waldinger MD, Zwinderman AH, Olivier B, SSRIs and ejaculation: a double – blind, randomized, fixed-dose study with paroxetine and citalopramJ Clin Psychopharmacol 2001 21(6):556-60. [Google Scholar]
[7]. Waldinger MD, Zwinderman AH, Olivier B, On-demand treatment of premature ejaculation with clomipramine and paroxetine: A randomized, double-blind fixed-dose study with stopwatch assessmentEur Urol 2004 46:510-16. [Google Scholar]
[8]. Bloom W, Fawcett DW, Male reproductive system. In A textbook of histology 1976 10th editionPhiladelphia, London, TorontoW. B. Saunders Company:805-55. [Google Scholar]
[9]. Ross MH, Pawlina W, Male reproductive SystemIn Histology A Text And Atlas with correlated cell and molecular biology. International edition 2008 6th editionNew YorkWolter Kluwer the point:784-829.(http://the Point.Iww.com/RossHistology_6e) [Google Scholar]
[10]. Ross MH, Pawlina W, Epithelial TissueIn Histology A Text And Atlas with correlated cell and molecular biology. International edition 2008 6th editionNew YorkWolter Kluwer the point:105-50.(http://the Point.Iww.com/RossHistology_6e) [Google Scholar]
[11]. Gustavo FG, Function of seminal vesicles and their role on male fertilityAsian J Androl 2001 3:251-58. [Google Scholar]
[12]. Pahdke AM, Samant NR, Deval SD, Significance of seminal fructose in male fertilityFertil Steril 1973 24:894-903. [Google Scholar]
[13]. Biswas S, Ferguson K, Stedronska J, Fructose and hormone levels in semen: their correlation with sperm counts and motilityFertil Steril 1978 30:200-04. [Google Scholar]
[14]. Higgins SJ, Burchell JM, Mainwaring WIP, Testosterone control of nucleic acid content and proliferation of epithelium and stroma in rat seminal vesiclesBiochem J 1976 160:43-48. [Google Scholar]
[15]. Tryphonas L, Hidiroglou M, Collins B, Reversal by Testosterone of Atrophy of Accessory Genital Glands of Castrated Male Sheep: A Histologic and Morphometric StudyVet Pathol 1979 16(6):710-21. [Google Scholar]
[16]. Giuliano F, Clèment P, Pharmacology for the Treatment of Premature EjaculationPharmacological reviews 2012 64(3):621-44. [Google Scholar]
[17]. Aggarwal A, Jethani SL, Rohatgi RK, Kalra J, Effects of Fluoxetine on Testis of Albino rats – A Histological AssessmentInternational Journal of Scientific & Engineering Research 2012 3(7):1-5. [Google Scholar]
[18]. Richardson D, Goldmeier D, Recommendations for the management of retarded ejaculation: BASHH special interest group for sexual dysfunctionInternational journal of STD and AIDS 2006 17:7-13. [Google Scholar]