Background: Fine needle aspiration cytology (FNAC) of lymph nodes is a simple, cost effective, out-patient procedure used for diagnosis of various causes of lymphadenopathies. In tuberculous lymphadenitis, it not only used for the cytological diagnosis but also used for other ancillary testing such as Ziehl- Neelsen staining and AFB Culture.
Aims: Our study was designed to evaluate the cytopathological pattern of FNAC aspirate of patients presenting with lymphadenopathy with special reference to tuberculous lymphadenopathy.
Materials and Methods: In this study all the patients referred to the cytopathology lab for FNAC of lymph nodes between January 2011 to June, 2013 were included. Out of 1050 patients presenting with lymphadenopathies, there were 550 cases of tuberculous lymphadenitis. The cytopathological findings of these 550 cases were analyzed.
Results: A female preponderance was noted in our study with maximum incidence in the 3rd decade. Cervical lymph nodes were the most common nodes to be involved. Gross examination of aspirate showed maximum cases (74.5.2%) of whitish material. Among the four cytological patterns on FNAC, maximum cases demonstrated caseous necrotic material with degenerated inflammatory cells. Ziehl- Neelsen staining showed overall AFB positivity of 44.54%. Maximum AFB positivity was seen in cases having caseous necrosis only.
Conclusion: FNAC is a simple, cost effective technique with high degree of accuracy in diagnosing Tubercular Lymphadenitis. Despite certain limitations and pitfalls, FNAC coupled with Ziehl- Neelson staining should be the 1st line investigation in cases with lymphadenopaty, in a developing country with high prevalence rate of tuberculosis.
Introduction
With the advent of HIV, there is a global upsurge of mycobacterial infection and tuberculosis has become a major cause of morbidity and mortality [1] . In India, the incidence of tuberculosis is high and tuberculosis is the leading cause of lymphadenopathy [2].
Superficial lymhadenopathies is the most common extra-pulmonary manifestation of Tuberculosis. Fine needle aspiration cytology (FNAC) is a safe, easy, cost effective and outpatient procedure to diagnose TB obviating the need for lymph node core needle or excision biopsy. Since, it carries a high degree of accuracy, it is used in diagnosis at the initial step as well as in follow up post treatment. For cytological diagnosis, we need to demonstrate epithelioid giant cell granulomas with or without caseous necrosis along with AFB staining for the definitive diagnosis. The present study aimed to access the accuracy of FNAC and its diagnostic relevance in developing countries like ours.
Materials and Methods
This prospective study was conducted in the Department of Cytopathology in our hospital. FNAC was carried out on all the patients, presenting with lymphadenopathy, between January 2011 to June 2013. The clinical data collected included patient’s age, gender, location of lymph nodes, clinical presentation, gross examination of aspirate, cytopathological examination of aspirate and special staining for AFB. The AFB culture on aspirated sample was not carried out as the facility for AFB culture was not available in the hospital.
Results
During this period, a total of 1050 FNAC were performed on patients with lymphadenopathy in the department. FNAC were done using 10ml syringe and 22 gauge needle under aseptic precautions. Air dried smears were stained with Giemsa stain and Haematoxylin and Eosin stains after fixation. In view of the high endemicity of tuberculosis in this part of the world, Ziehl- Neelsen staining was also carried out.
Amongst the 1050 patients presenting with lymphadenopathy, FNAC revealed 85 (8.1%) malignant lesions and 965 (91.9%) benign. The malignant lesions were excluded from the study. Of the 965 cases of benign lymphadenopathy , tuberculosis lymphadenitis was the predominant lesion comprising of 550 (56.99%) cases, followed by reactive lymphoid hyperplasia 340 (35.23%) cases, acute suppurative lymphadenitis 40 (4.14%) and 35(3.6%) chronic inflammatory lymphadenitis [Table/Fig-1] .
Among the 550 cases of tuberculosis lymphadenitis, the maximum incidence of tubercular lymphadenitis was noted in the 3rd decade followed by the 2nd decade. The youngest patient in the series was a three months old infant whereas the oldest patient in the series was 77-years-old. Mean age of presentation of tuberculosis lymphadenopathy in this study was 35.2 y. There was female predominance, with male to female ratio being 1:1.38. The female’s preponderance was particularly very high in 2nd decade of life whereas it was male preponderance in the 3rd decade of life. In middle age group and in elderly population, there was no significant difference in the incidence of disease amongst males and females.
Tuberculosis lymphadenopathy presented mostly as solitary lesion. The cervical nodes were the most frequently involved group (70%) whereas inguinal lymphadenopathy was least common (2.54%)[Table/Fig-2] .
According to nature of aspirated material, whitish material was obtained in 410 (74.5%) cases, while in 50 (9.1%) cases, the aspirate was cheesy and blood mixed in 90 (16.4%) cases.
According to cytology, we classified the smears into four categories [Table/Fig-3] .
1. Smears showing only caseous necrosis [Table/Fig-4].
2. Smears showing caseous necrotic material with degenerated inflammatory cells [Table/Fig-5].
3. Smears showing caseous necrotic material with epithelioid giant cell granulomas and giant cells [Table/Fig-6].
4. Smears showing epithelioid giant cell granulomas without necrosis [Table/Fig-7].
Maximum AFB positivity (94.1%) [Table/Fig-8] was seen in smears from lesions having only caseous necrosis followed by smears from caseous necrotic material with degenerated inflammatory cells (52.8%). Least positivity (2.56%) was seen in smears prepared from lesions with granulomas without necrosis. The overall AFB positivity in the series was 44.54% [Table/Fig-3].
Discussion
In India, tuberculosis lymphadenopathy is one of the most common type of lymphadenopathy encountered [3-6]. In the current series, among benign causes of lymphadenopathy in 965 cases, tuberculosis lymphadenopathy was the commonest (550 cases) (56.99%). This finding was comparable with the other studies [2]. However, study by Chawla et al., [7] showed relatively lower incidence of tubercular lymphadenopathy (31.7%). In some western studies the incidence of tuberculosis lymphadenitis was very low (1.6%) [8] . The high incidence of tuberculosis in our set up may be due to the fact that our hospital caters to a large population of low socioeconomic strata.
The present study showed the maximum incidence of tuberculosis lymphadenitis in the 3rd decade followed by 2nd decade. The mean age of presentation was 35.2 y, with a total of 60.3% cases in the 2nd & 3rd decade of life. Similar pattern of age distribution has also been in some studies [2,7]. Mohapatra and Janmeja [9] had reported the maximum incidence seen in 2nd decade followed by 3rd decade. The youngest patient in our series was a three months old child while the oldest one was 77 y. The disease appears to be less common in extremes of age group. Narang et al., [10] had reported minimum age of 4 years with maximum being 70 y.
The present study, found that the females were more commonly affected than males (male to female ratio was 1:1.38). The higher incidence of disease among females may be due to the low immunity of Indian females, particularly those belonging to low socioeconomic strata and those in reproductive age group. Paliwal et al., [11], Khajuria et al., [2], Narang et al., [10] , Mohapatra and Janmeja [9] also noted the female preponderance in their studies.
According to site, the cervical lymph nodes were most commonly involved i.e. 70%, with solitary lymphadenopathy being the commonest presentation. Cervical nodes were also found to be most frequently involved in other studies [2][11] In our series, patients most commonly presented with single nodes (451 cases i.e. 81.6%), while 18.4% patients presented with multiple cervical nodes. Paliwal et al., [11] had reported 7.2% patients presented with bilateral cervical nodes and 19.2% with multiple unilateral nodes.
According to gross nature of aspirate, maximum were (74.5%) followed by blood-mixed (9.1 %) and cheesy (16.4%). Metre and Jayaram [12] in their study had divided the aspirate grossly as blood mixed, cheesy and purulent.
For cytodiagnosis of tuberculosis lymphadenopathy, we need to demonstrate epithelioid giant cell granulomas, langhans giant cells with or without caseous necrosis along with demonstration of AFB positivity by ZN staining. Studies by Gomes et al., [13], Das et al., [14] also followed the same cytological parameters for diagnosis of tubercular lymphadenitis.
In our study, based on cytological findings, we categorized the lesions into four categories: a) Cases with only caseous necrosis, b) Cases with caseous necrotic material and degenerated inflammatory cells, c) Cases with caseous necrotic material and epithelioid giant cell granulomas and giant cells and d) cases with reactive lymphoid cells and epithelioid giant cell granulomas [Table/Fig-3].
In our series of 550 cases, among the four cytological patterns, maximum cases were having caseous necrotic material with degenerated inflammatory cells without epithelioid cell granulomas (34.4%), followed by epithelioid cell granulomas without necrosis (28.4%), caseous necrotic material with epithelioid giant cell granulomas and giant cells (21.8%) and caseous necrotic material (15.4%).
Paliwal et al., [11], in their series of 176 cases of tubercular lymphadenopathy, had the maximum cases having cytopathological picture of necrosis only without epithelioid granulomas (39.2%), followed by cases having polymorphs with necrosis with or without epithelioid granulomas (30.1%). Their series had 16.4% cases with epithelioid cell granulomas with necrosis whereas least common cytopathological finding of epitheloid granuloma without necrosis was seen in 14.3% cases. Gupta et al., [11] had much higher percentage of cases showing epithelioid cell clusters with or without langhans giant cells with necrosis (50.35%).
In our study overall AFB positivity was 44.54% while Paliwal et al., [1] had 71% positivity and 59.5% cases by Bezabih et al., [15]. Low incidence of AFB positivity in our study may be due to combined maximum percentage (62.8%) of cases having epithelioid cell granulomas with necrosis and cases with epitheloid cell granulomas without necrosis unlike series of Paliwal et al., [11] where this combined percentage was only 30.7%. Das Gupta et al., [16] in their series of 180 cases of cervical lymphadenopathy reported 45.65% AFB positivity which is similar to our series of 550 cases. On the other hand, a very low positivity rate of AFB (19.6% cases) on Z.N Staining was reported by Aggarwal et al., [17] in their series of 138 cases. But, in their series as well, the lymph nodes aspirate culture was positive for Mycobacterium tuberculosis in 40.6% cases. This, significant rise in number of cases confirmed on AFB culture over the method of AFB detection by Z.N. Staining in their series, could not be corroborated in our study as AFB culture was not carried out in our study.
In view of high endemic prevalence in India, when AFB is negative in presence of epithelioid granulomas with or without necrosis possibility of tubercular etiology is always very high and the patient is advised for further investigations and follows up. The same procedure was followed by Das et al., [14].
Studies have shown that chance of finding AFB is higher with cold abscess formation i.e. cases showing necrosis and degenerated inflammatory cells [9]. Maximum AFB positivity of 94.1% was seen in cases having caseous necrosis only and least with epithelioid cell granulomas without necrosis i.e. 2.54%. Similar observations were also reported by Paliwal et a., [11], Bezabih et al.,[15] , and Gupta et al., [5] .
Highest AFB positivity in our series was with cheesy aspirate (94.1%) followed by purulent aspirate (50.8%). While study by Paliwal et al., [11] and Ahmed et al., [18] revealed maximum AFB positivity in purulent aspirate with percentage of 61.6% and 68.8% respectively.
In our study care was taken while screening AFB smears. Care was also taken in screening AFB smears of metastatic carcinomas that yielded cheesy or purulent aspirate with scanty viable carcinoma cells that are prone for misdiagnosis.
Our study had 20 cases as defaulters of treatment and 12 cases that had completed their treatment but had persisting nodes or new emerging nodes. In all the 20 defaulters, the FNAC smears of the lymph node aspirate was AFB positive, whereas in the 12 cases who had received full course of anti- tubercular treatment, the FNAC smear of the aspirate was AFB negative.
Although, FNAC is a good method to diagnose tuberculosis lymphadenopathy, this procedure has several limitations and pitfalls as observed in our series.
1. Sampling or technical error: Four cases on initial aspiration yielded only blood cells with scanty lymphoid cells with or without scattered epithelioid cells. Repeat aspiration in these cases yielded purulent material in two cases and blood mixed in the other two. The first two cases were diagnosed Tuberculosis lymphadenitis with AFB positive while the other two were Granulomatous picture with AFB negative. In view of the fact that FNAC is a blind procedure, it is possible that needle during the initial aspiration may not have reached the exact pathological site which leads to the inadequate aspirate. So, it is suggested that a repeat aspiration may be carried out in cases where there is high suspicion of tuberculosis, based upon clinical history, family history or size and other features of lymphnodes.
2. Purulent or Cheesy aspirate in cases of metastatic squamous cell carcinoma with central degeneration may lead to interpretative error: Sometimes metastatic carcinomas with degeneration yields only necrotic material that is cytologically similar to caseous necrosis of TB. Only the careful screening of the smears with search for viable tumour cells and AFB screening helped us reaching at the correct diagnosis. In some cases repeat FNA from the periphery of the node yielded viable clusters of tumour cells and thus helps ruling out tubercular pathology.
3. FNAC versus Histopathology: One patient aged 8 y was having single cervical node of 2cm size. It was diagnosed as Reactive hyperplasia node even on repeat aspiration. But due to its size and clinical history of having fever of one month duration, he was advised biopsy. Histopathology of the excision biopsy of the node yielded epitheliod cell granulomas near the subcapsular sinus with florid reactive hyperplasia of the other areas of the node. The failure of FNAC in diagnosing this case was due to the blind procedure of FNAC where the cytologist failed to reach the pathological site due to the limited focal involvement of the node.
In another 25 y female presenting with single cervical node of size 2cm.x 1.5 cm, of one month duration, FNAC yielded blood mixed aspirate. Smear showed mostly reactive lymphoid cells with few scattered epitheloid cells and AFB was negative. This patient was subjected to biopsy. Histopathological examination of the lymph node in this case showed epithelioid giant cell granulomas with reactive lymphoid hyperplasia of the other areas.
Spectrum of benign lymphadenopathies (n=965)
| Type of lesions | No of cases | % of cases |
| Tubercular | 550 | 56.99% |
| Reactive Lymphoid Hyperplasia | 340 | 35.23% |
| Acute Suppurative Lymphadenopathy | 40 | 4.14% |
| Chronic Inflammatory Lymphadenopathy | 35 | 3.6% |
Distribution of sites of tuberculosis lymphadenitis: (n=550)
| Anatomical Region | No of cases | % |
| Cervical | 385 | 70% |
| Supraclavicular | 75 | 13.65% |
| Axillary | 37 | 6.72% |
| Inguinal | 14 | 2.54% |
| Submandibular | 22 | 4% |
| Submental | 17 | 3.09% |
Distribution of cases according to cytology & AFB positivity: (n=550)
| Cytology in Smears | AFB positive Number of cases (%) | AFB negative Number of cases (%) | Total Number of Case (%) |
| Only caseous necrosis | 80 (94.1%) | 5 (5.9%) | 85 (15.4%) |
| Caseous necrotic material with degenerated inflammatory cells | 100 (52.8%) | 89 (47.2%) | 189 (34.4%) |
| Caseous necrotic material with epithelioid giant cell granulomas and giant cells | 61 (50.1%) | 59 (49.9%) | 120 (21.8%) |
| Epithelioid giant cell granulomas without necrosis | 4 (2.56%) | 152 (97.44%) | 156 (28.4%) |
| Total Cases | 245 (44.54%) | 305 (55.46%) | 550 (100%) |
Cytological smear showing caseous necrotic material. (Haematoxylin and Eosin, 40x10X)
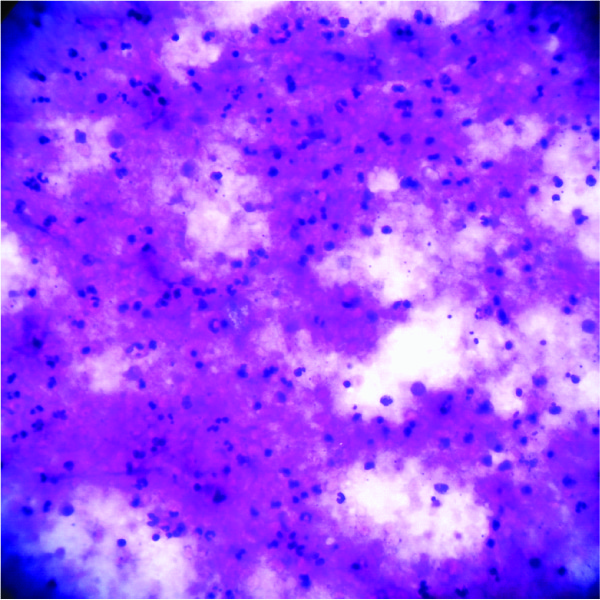
Cytological smear showing degenerated inflammatory cells and necrotic (Haematoxylin and Eosin, 40x10X)
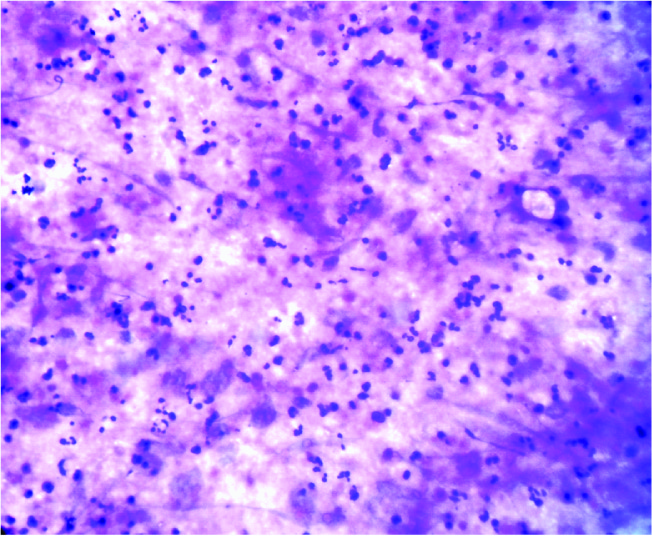
Cytological smear showing epithelioid giant cell granuloma in a necrotic background (Haematoxylin and Eosin, 40x10X)
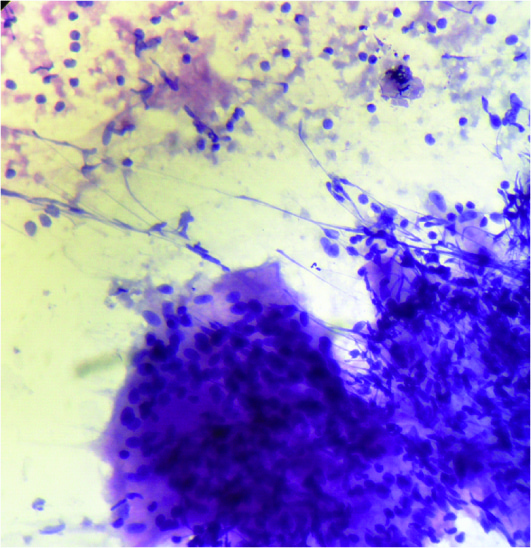
Cytological smear showing giant cell with epithelioid cell granuloma and Langhans giant cell, without necrosis (Giemsa stain, 40x10X)

Smear shows singly lying and clusters of ABF (Zeihl and Nelson Stain 100x10 X)
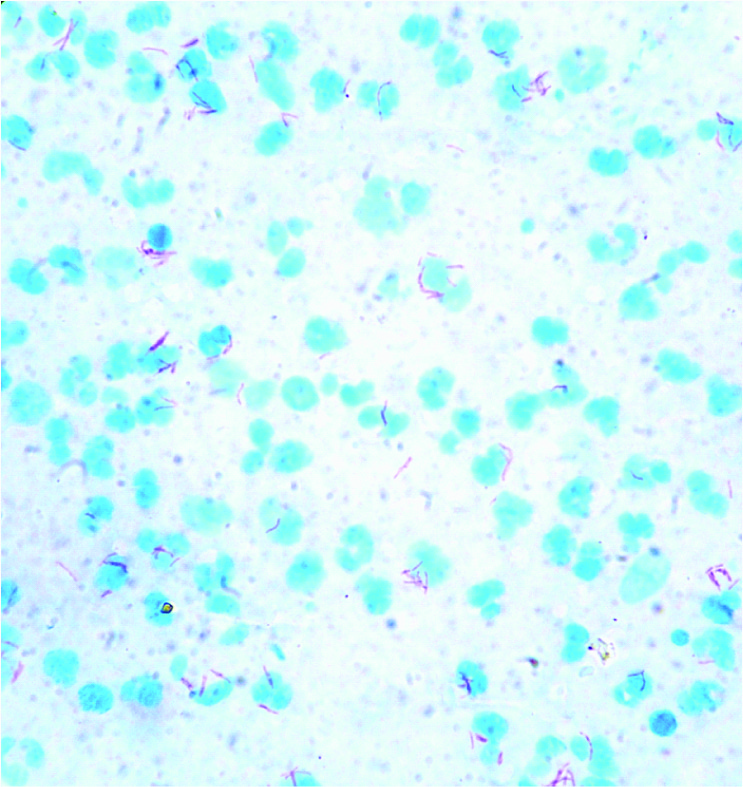
Conclusion
FNAC is a reliable, quick and economical investigating modality in tubercular lymphadenopathy. Despite certain limitations and pitfalls, it provides a high degree of accuracy in diagnosing tuberculosis. Prompt diagnosis is helpful in reducing morbidity and mortality of tuberculosis. The demonstration of AFB in the FNAC smear is a gold standard for diagnosing tuberculosis. This can be easily done by Z.N. Staining. Wherever facilities of AFB culture are available, they may be used for detection of AFB. The FNAC smears showed AFB positivity on Z.N. staining in 245 (44.54%) cases. On the other hand, rests of the 305 cases were negative for AFB, but the cytological pattern seen on FNAC smears was strongly suggestive of tuberculosis. We feel that in a developing country with high prevalence rate of tuberculosis, FNAC coupled with Z.N. staining should be the 1st line investigation in cases with lymphadenopathy. After cytopathological diagnosis, decision regarding AFB culture, biopsy or other relevant investigations can be taken up case to case if necessary.
[1]. K Das Dilip, Fine-Needle Aspiration Cytology in the Diagnosis of Tuberculosis Lesions.Laboratory Medicine. 2000 31(11):625-32. [Google Scholar]
[2]. Ruchi Khajuria, KC Goswami, K Singh, VK Dubey, Pattern of Lymphadenopathy on Fine Needle Aspiration Cytology In Jammu.JK Science. 2006 190(3):158-60. [Google Scholar]
[3]. RR Prasad, R Narasimhan, V Sankran, AJ Veliath, Fine needle aspiration cytology in the diagnosis of superficial lymphadenopathy: an analysis of 2418 cases.Diagno Cytol. 1996 15(5):382-86. [Google Scholar]
[4]. PC Paul, BK Goswami, S Chakrabarty, A Giri, R Pramanik, Fine needle aspiration cytology of lymph nodes-An institutional study of 1448 cases over a five year period.J Cytology. 2004 21:187-90. [Google Scholar]
[5]. AK Gupta, M Nayar, M Chandra, Critical appraisal of fine needle aspiration cytology in tuberculosis. Acta Cytol. 1992 36(3):391-94. [Google Scholar]
[6]. CS Bhaskran, GH Kumar, M Sreenivas, K Rajni, G Rao, CA Aruna, Fine needle aspiration cytology review of 1731 cases. Ind J Pathol Microbiol. 1990 33:87-97. [Google Scholar]
[7]. Nitin Chawla, Sanjeev Kishore, Sandip Kudesia, FNAC of Lymph Node Disorders. Indian Medical Gazette. 2012 :312-15. [Google Scholar]
[8]. TS Kline, V Khannan, IK Line, Lymphadenopathy and aspiration biopsy cytology review of 376 superficial lymphnodes. Cancer. 1984 54:1076-81. [Google Scholar]
[9]. Raghab Mohapatra Prasanta, Kumar Janmeja Ashok, Tuberculosis Lymphadenitis.JAPI. 2009 57:585-90. [Google Scholar]
[10]. RK Narang, S Pradhan, RP Chaturvedi, S Sheridan, Place of Fine Needle Aspiration Cytology In The Diagnosis of Lymphadenopathy. Ind J Tub. 1990 37:29-31. [Google Scholar]
[11]. Nidhi Paliwal, Sapna Thakur, Shalini Mullick, Gupta Kumud, FNAC In Tuberculosis Lymphadenitis : Experience From A Tertiary Level Referral Centre.Indian J Tuberc. 2011 58:102-07. [Google Scholar]
[12]. S Metre, G Jayaram, Acid-fast bacilli in aspiration smears from tuberculosis lymph nodes: an analysis of 255 cases.Acta Cytol. 1987 31:17-19. [Google Scholar]
[13]. I Gomes, E Trindade, O Vidal, Diagnosis of sputum smear–negative forms of pulmonary tuberculosis by transthoracic fine needle aspiration.Tubercle. 1991 72:210-13. [Google Scholar]
[14]. DK Das, JN Pank, KL Chachra, Tuberculosis lymphadenitis: correlation of cellular components and necrosis in lymph node aspirate with AFB positivity and bacillary count.Indian J Pathol Microbiol. 1990 33:1-10. [Google Scholar]
[15]. M Bezabih, DW Mariam, SG Selassie, Fine needle aspiration cytology of suspected tuberculosis lymphadenitis.Cytopathology. 2002 13(5):284-90. [Google Scholar]
[16]. A Dasgupta, RN Ghosh, AK Poddar, Fine needle aspiration cytology of cervical lymphadenopathy withspecial reference to tuberculosis.J Indian Med Assoc. 1994 92(2):44-46. [Google Scholar]
[17]. P Aggarwal, JP Wali, S Singh, R Handa, N Wig, A Biswas, A clinico-bacteriological study of peripheral tuberculosis lymphadenitis.J Assoc Physicians India. 2001 49:808-12. [Google Scholar]
[18]. SS Ahmad, S Akhtar, K Akhtar, S Naseem, T Mansoor, S Khalil, Incidence of tuberculosis from study of fine needle aspiration cytology in lymphadenopathy and acid fast staining.Ind J Community Medicine. 2005 30(2):63-65. [Google Scholar]